Your Location:Home >10241-05-1
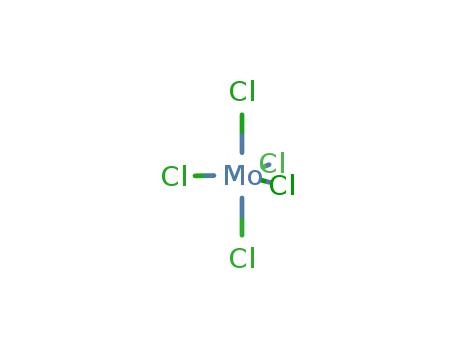

Product Details
|
Preparation |
Molybdenum pentachloride may be prepared by heating molybdenite in chlorine. Sulfur chloride formed in the reaction is removed by distillation: 2MoS2 + 7Cl2 → 2MoCl5 + 2S2Cl2 Also, the compound may be prepared by the action of chlorine on molybdenum metal at elevated temperatures (500°C): 2Mo + 5Cl2 → 2MoCl5 The pentachloride may be obtained from the tetrachloride, MoCl4. The latter, when heated in a sealed tube sublimes, and upon cooling, disproportionates to MoCl5 and the trichloride, MoCl3: 2MoCl4 → MoCl5 + MoCl3 |
|
Air & Water Reactions |
MOLYBDENUM(V) CHLORIDE may react with water to produce corrosive hydrochloric acid and toxic fumes. |
|
Reactivity Profile |
MOLYBDENUM(V) CHLORIDE is a corrosive, hygroscopic solid, on contact with water or steam MOLYBDENUM(V) CHLORIDE decomposes to form hydrochloric acid. When heated to decomposition MOLYBDENUM(V) CHLORIDE emits toxic fumes of molybdenum chlorides and metallic molybdenum [Lewis, 3rd ed., 1993, p. 892]. Explodes on contact with finely divided sodium [Berry D. H., Chem. Eng. News, 1989, 67(47), p. 2]. Reaction with finely divided sodium sulfide is violent, may lead to autoignition [Kaner, R. B., Nature, 1991, 349, p. 510]. |
|
Hazard |
Irritant. |
|
Health Hazard |
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. |
|
Safety Profile |
A poison. A corrosive irritant to skin, eyes, and mucous membranes. Reacts with moisture to form hydrochloric acid. When heated to decomposition it emits toxic fumes of Mo and Cl-. |
|
Physical Properties |
Greenish-black monoclinic crystals or dark red as liquid or vapor; paramagnetic; hygroscopic; critical temperature 577°C; critical volume 369 cm3/mol; soluble in dry ether, dry alcohol and many other organic solvents; reacts with water. |
InChI:InChI=1/5ClH.Mo/h5*1H;/q;;;;;+5/p-5/rCl5Mo/c1-6(2,3,4)5
Companies adhering to the "science and technology aim, quality first, customer first, honest and trustworthy" business philosophy, in line with the "pragmatic, integrity, unity, innovation" spirit of enterprise, forge ahead, constantly pursuing higher levels of product quality and service. Warmly welcome customers at home and abroad to establish mutual trust, mutual benefit, and stable cooperation!
Matrix-isolation studies have been carri...
The reduction of MoCl5 with metallic tin...
MoCl4, ReCl4, and ReCl5 react with PCl5 ...
The compound Mo2Cl8O was formed as a min...
chlorine


molybdenum

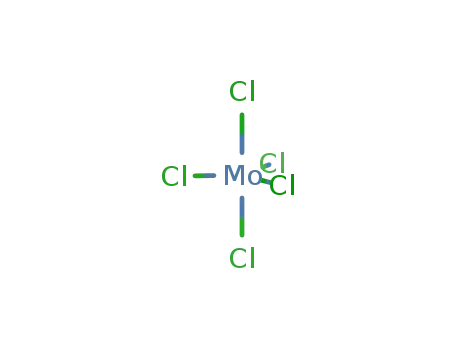
molybdenum(V) chloride

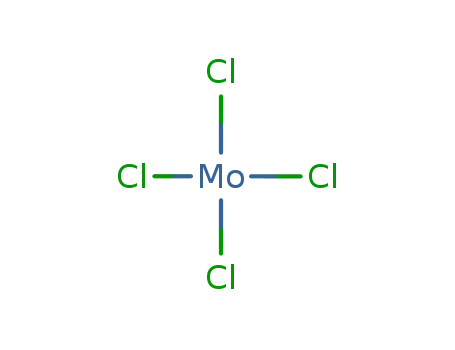
molybdenum(IV) chloride
| Conditions | Yield |
|---|---|
|
In neat (no solvent); introduction of Cl2-gas (8-9 Torr/20 min/1.8 l/h) through purified (90min/H2 stream/1000°C) Mo powder (860 - 1160°C);; extraction of MoCl5 (CCl4); extraction MoCl4 (diethylether or water); further extraction (ethanol or water);;
|
0-10 |
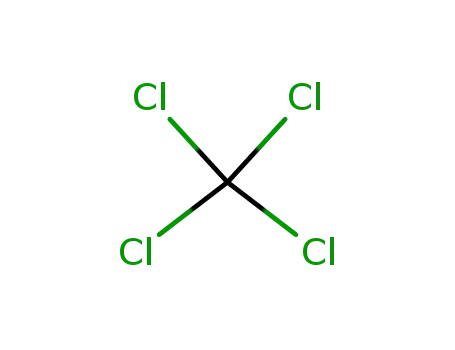
tetrachloromethane

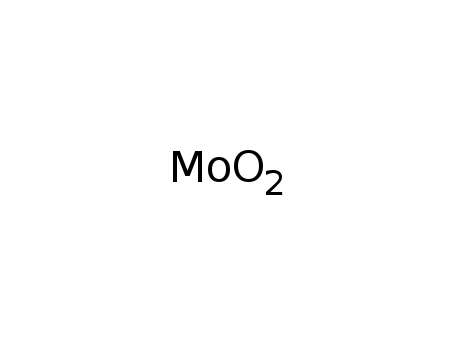
molybdenum(IV) oxide


molybdenum(V) chloride


molybdenum(IV) chloride
| Conditions | Yield |
|---|---|
|
With pyrographite; mass ratio MoO2:C = 5:1; mixt. heated at 300°C in a flow of N2 loaded with CCl4; absence of moisture and oxygen; further by-product: Mo oxide chloride; removal of MoCl5 and Mo oxide chloride by vac. distn.;
|
<1 |
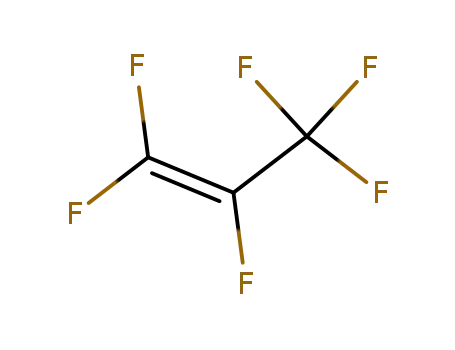
perfluoropropylene
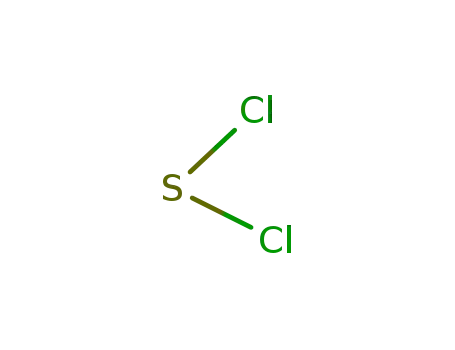
sulfur dichloride
tetrachlorosilane

tetrachloromethane
molybdenum(VI) tetrachloride oxide
dioxomolybdenum(VI) dichloride

molybdenum(IV) chloride

molybdenum
CAS:123-00-2
CAS:12027-06-4