
Product Details
|
Synthesis |
(1S)-(+)-Camphor-10-sulphonic acid can be obtained from natural camphor by brominated sulfonation . |
|
Purification Methods |
Crystallise the acid from ethyl acetate and dry it under vacuum (deliquescent). [Loudon J Chem Soc 823 1933, Komppa J Prakt Chem 162 19 1943, Beilstein 11 IV 642.] See above for RS-isomer. |
InChI:InChI=1/C10H16O4S/c1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h7H,3-6H2,1-2H3,(H,12,13,14)/p-1/t7-,10+/m0/s1
Hangzhou Ocean Chemical Co., Ltd. is a chemical supplier that provides stable product quality, unique technical support, and high-quality service for global customers, Headquarters is located in Hangzhou with a superior entrepreneurial environment and business climate.
Reported here is a simple and practical ...
New cyclofructan-6 (CF6)-based chiral st...
The Wagner-Meerwein domino rearrangement...
Ecto-5'-Nucleotidase inhibitors have gre...
![1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-one](/upload/2024/11/42f3b6b7-9369-4ee7-a993-e4645fa28241.png)
1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-one

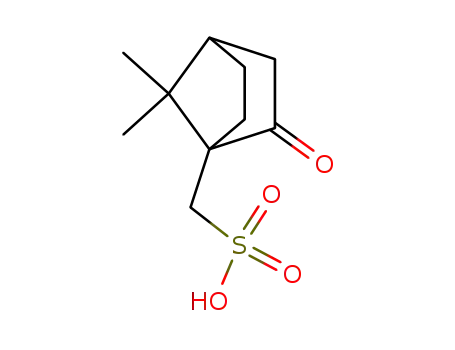
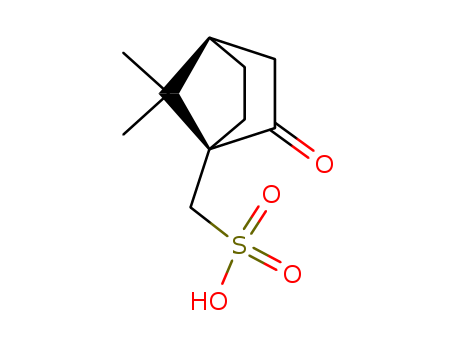
camphor-10-sulfonic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; acetic anhydride;
|

![7,7'-di-tert-butyl-5,5'-dimethyl-[3,3']bibenzofuranylidene-2,2'-dione](/upload/2024/11/419b2078-7223-43df-9030-74c95e79d63d.png)
7,7'-di-tert-butyl-5,5'-dimethyl-[3,3']bibenzofuranylidene-2,2'-dione


camphor-10-sulfonic acid
| Conditions | Yield |
|---|---|
|
|
38% |
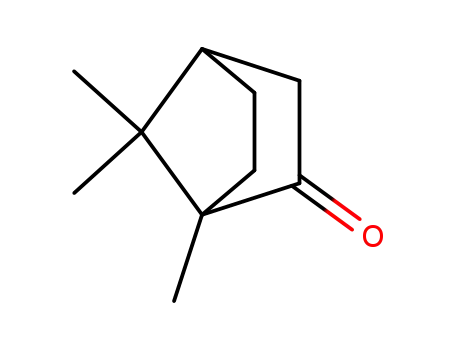
1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-one
brucine
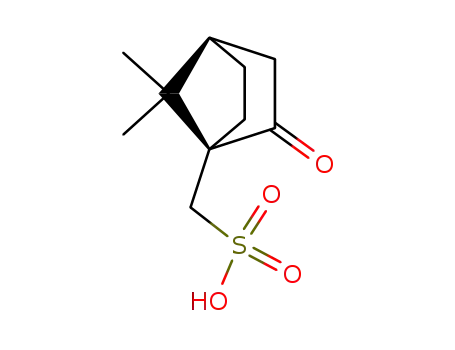
10-camphorsufonic acid
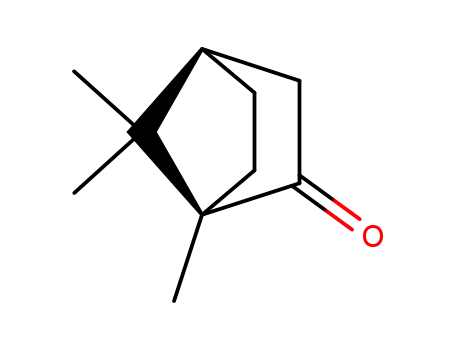
(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
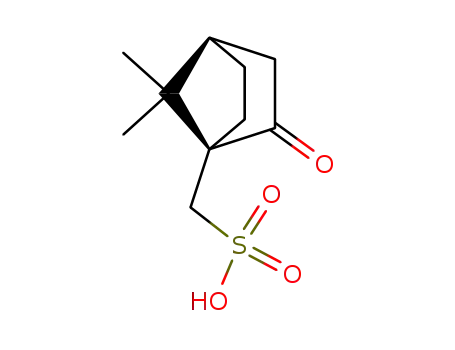
(1S)-10-camphorsulfonic acid
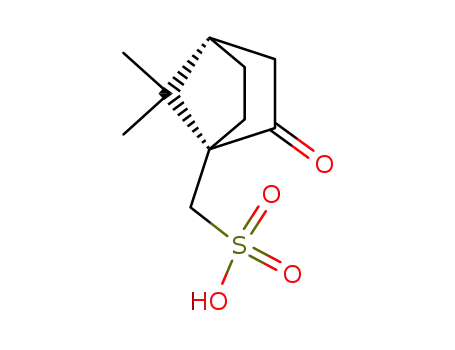
(R)-10-camphorsulfonic acid
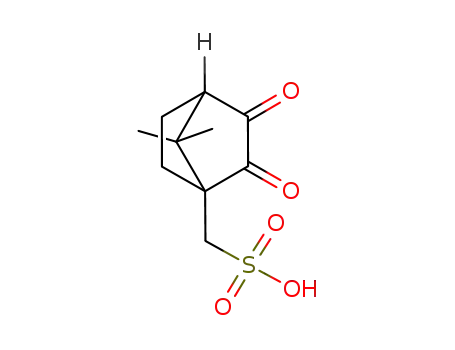
7,7-dimethyl-2,3-dioxo-bicyclo[2.2.1]heptane-4-methanesulfonic acid
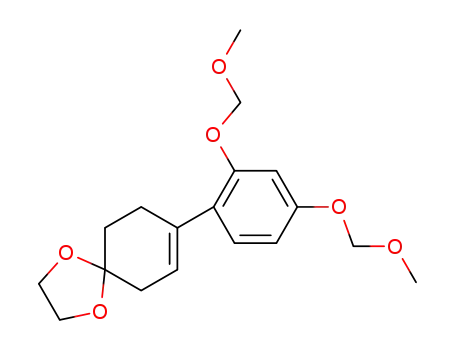
8-[2,4-Bis(methoxymethoxy)phenyl]-1,4-dioxaspiro[4.5]dec-7-ene
CAS:7365-82-4
CAS:10191-18-1
CAS:3470-98-2
CAS:18851-33-7