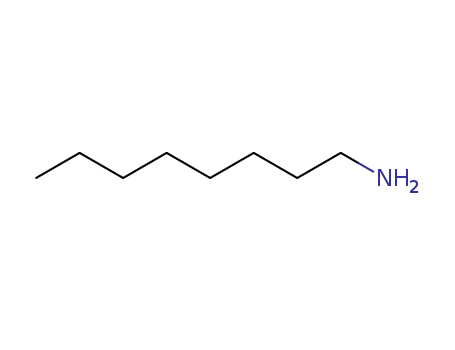

Product Details
| Product Name | n-Octylamine |
| Alias | 1-Aminooctane, n-Octylamine, Caprylamine |
| English | |
| Molecular formula | C8H19N |
| Molecular weight | 129.24 |
| CAS No | 111-86-4 |
| EINECS | |
| Specification | Assay(GC)99% Water < 0.3% color < 30 # (APHA) |
| Appearance traits | |
| Use | This product is mainly used as fungicide, pesticide, surfactant and pharmaceutical intermediates. |
| Package | 160kg/drum |
| Other product information | Properties: Appearance:Colourless oil liquid, Melting point:−4°C Boiling point:179.4°C Density:0.782 g/ml at 25 °C Vapour pressure:1 mm hg ( 20 °C) Refractive index:N20/D 1.432(lit.) Flash(ing) point:145 °F Water soluble properties:0.2 g/L (25 ºC) |
|
Production Methods |
Specific uses were not located in the literature. 1-Octylamine is manufactured under the tradenameArmeen8D(Armak). |
|
Synthesis Reference(s) |
Chemistry Letters, 7, p. 1057, 1978The Journal of Organic Chemistry, 47, p. 4327, 1982 DOI: 10.1021/jo00143a031Tetrahedron Letters, 11, p. 3411, 1970 |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
OCTANAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
|
Flammability and Explosibility |
Flammable |
|
Definition |
ChEBI: An 8-carbon primary aliphatic amine. |
|
General Description |
A yellow liquid with an ammonia-like odor. Insoluble in water and less dense than water. Hence floats on water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals. |
InChI:InChI:1S/C8H19N/c1-2-3-4-5-6-7-8-9/h2-9H2,1H3
Companies adhering to the "science and technology aim, quality first, customer first, honest and trustworthy" business philosophy, in line with the "pragmatic, integrity, unity, innovation" spirit of enterprise, forge ahead, constantly pursuing higher levels of product quality and service. Warmly welcome customers at home and abroad to establish mutual trust, mutual benefit, and stable cooperation!
Mild and efficient preparation of alkyl ...
A novel hydrogen-bonded supramolecular s...
-
Borohydride exchange resin (BER) - nicke...
Direct and regiospecific amino-functiona...
Various bacterial cells were tested to i...
Ultrasonic relaxation absorption has bee...
Fatty amines represent an important clas...
A process for catalytic amination of an ...
A process for producing a supported cata...
Amines are an important class of compoun...
complex of α-cyclodextrin with n-octylamine


n-Octylamine

alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
With phosphate buffer; In water-d2; at 25 ℃; Equilibrium constant; Thermodynamic data; standard molar enthalpy ΔrH0, standard molar Gibbs energy ΔrG0, standard molar entropy ΔrS0;
|
|
|
In alkaline aq. solution; at 25 ℃; pH=11.60; Equilibrium constant;
|
9-hydroxyimino-heptadecanoic acid

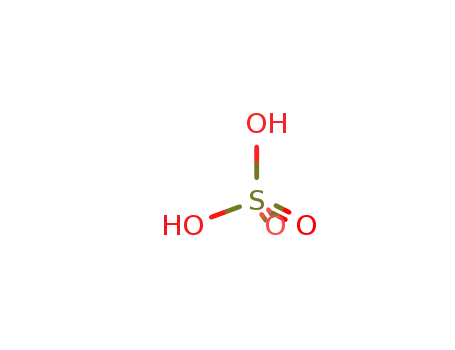
sulfuric acid

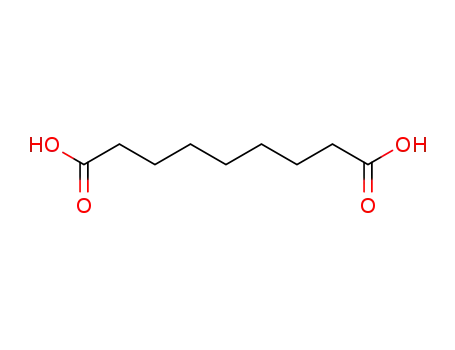
azelaic acid


n-Octylamine

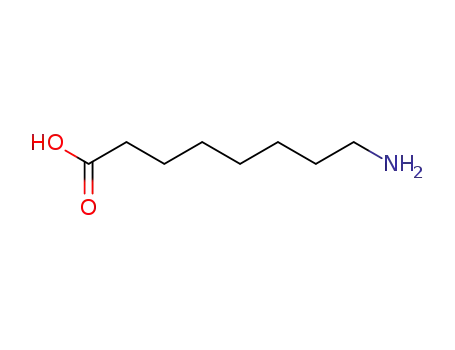
8-Aminooctanoic acid
| Conditions | Yield |
|---|---|
|
Erhitzen mit konz. Salzsaeure auf 180grad.Hydrolysis;
|
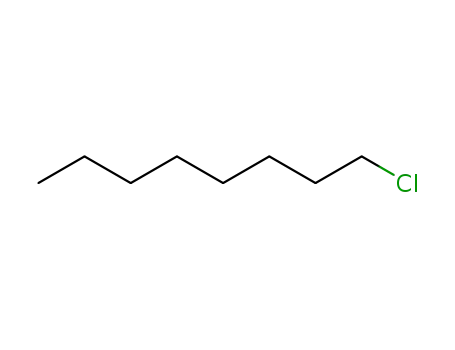
n-chlorooctane

1-Iodooctane
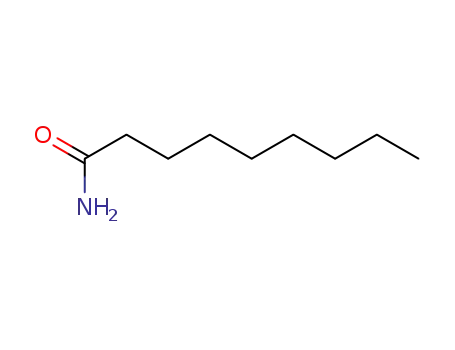
nonanamide
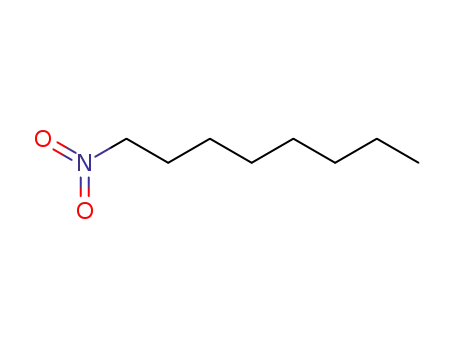
1-nitrooctane
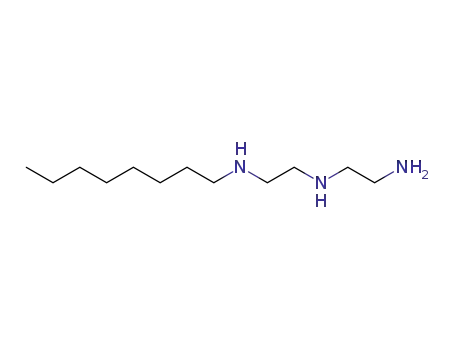
(2-amino-ethyl)-(2-octylamino-ethyl)-amine
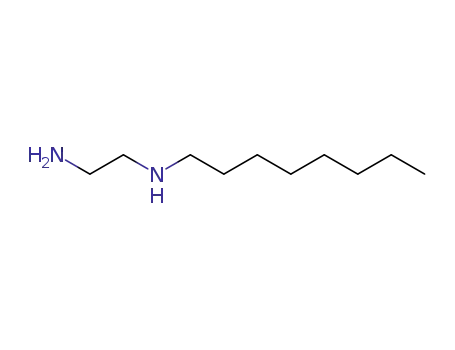
N-n-Octyl-diaminoethan-1,2
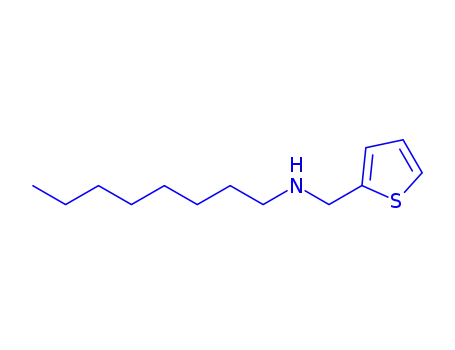
octyl-[2]thienylmethyl-amine
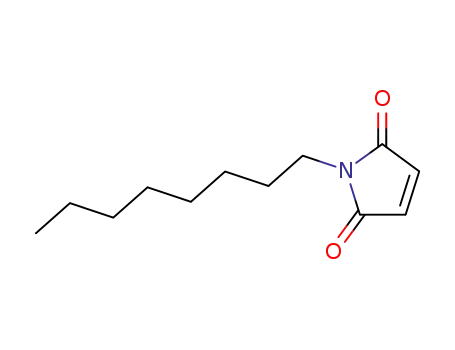
N-octylmaleimide
CAS:80-70-6
CAS:10061-68-4
CAS:15875-13-5
CAS:1116-76-3