
Product Details
| Product Name | N, N-dimethylbenzamine |
| Alias | dimethylbenzylamine;N,N-Dimethylbenzylam;N-BENZYLDIETHYLAMINE |
| English | |
| Molecular formula | C9H13N |
| Molecular weight | 135.21 |
| CAS No | 103-83-3 |
| EINECS | |
| Specification | Content > 99% Water < 0.5% Colour and lustre is < 100 # (APHA) |
| Appearance traits | properties: it is colorless to light yellow transparent liquid at normal temperature and has a strong ammonia flavor. |
| Use | N, N - dimethyl benzylamine is a standard lumbar soft bubble and hard foaming catalyst, as well as organic synthesis and acid neutralizing agent and dehydrogenation catalyst. |
| Package | 180 kg/barrels or according to customer requirements |
| Other product information | Density 0.9; Melting point - 75 oC; Boiling point 183-184 oC; Refractive index 1.5-1.502; Flash point 54 oC; Water soluble 8 g/L (20 oC); |
|
Preparation |
25% Aqueous Dimethylamine, 1088 grams Benzyl Chloride, 126.6 grams In the apparatus of Example 1, the benzyl chloride was added dropwise over a two-hour period to the amine (molar ratio 1 to 6) at a rate sufficient to maintain the temperature below 40°C. Stirring was continued at room temperature for an additional hour to insure completion of the reaction denoted by the equation below. Thereafter the reaction mixture was cooled in a separatory funnel while standing in a refrigerator maintained at 5° C. and separated into two layers. The upper oily layer, weighing 111.5g, was removed and steam distilled until no further oleaginous component was observed in the distillate as it came over. The crude distillate was found to contain 103.5g of N,N-dimethylbenzylamine (76.1% of theory), 3.3g of dimethylamine and no quaternary salts. The dimethylamine was distilled off below 29°C under atmospheric pressure from the N,N-dimethylbenzylamine (bp 82°C/18mmHg). |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 40, p. 531, 1975 DOI: 10.1021/jo00892a043Synthetic Communications, 6, p. 539, 1976 DOI: 10.1080/00397917608063545 |
|
Air & Water Reactions |
Flammable. Slightly soluble in water. |
|
Reactivity Profile |
N,N-Dimethylbenzylamine neutralizes acids on exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. May attack some plastics [USCG, 1999]. |
|
Health Hazard |
Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomitting. |
|
Fire Hazard |
Special Hazards of Combustion Products: Toxic vapors are generated when heated. |
|
Flammability and Explosibility |
Flammable |
|
Safety Profile |
Poison by ingestion. Moderately toxic by inhalation and skin contact. Corrosive. A severe eye and skin irritant. Flammable when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NOx |
|
Purification Methods |
Dry the amine over KOH pellets and fractionate it over Zn dust in a CO2—free atmosphere. It has a pKa2 5 of 8.25 in 45% aqueous EtOH. Store it under N2 or in a vacuum. The picrate has m 94-95o, and the picrolonate has m 151o (from EtOH). [Skita & Keil Chem Ber 63 34 1930, Coleman J Am Chem Soc 55 3001 1933, Devereux et al. J Chem Soc 2845 1957.] The tetraphenyl borate salt has m 182-185o. [Crane Anal Chem 28 1794 1956, Beilstein 12 IV 2161.] |
|
General Description |
A colorless to light yellow liquid with an aromatic odor. Slightly less dense than water and slightly soluble in water. Flash point approximately 140°F. Corrosive to skin, eyes and mucous membranes. Slightly toxic by ingestion, skin absorption and inhalation. Used in the manufacture of adhesives and other chemicals. |
InChI:InChI=1/C9H13N/c1-10(2)8-9-6-4-3-5-7-9/h3-7H,8H2,1-2H3/p+1
Our main markets are China, the United States, Europe, Korea, and Japan. The main business scope includes electronic chemicals, liquid crystal intermediates, high-purity metal compounds, pharmaceutical intermediates, special chemicals, and so on.
-
B(C6F5)3 was proven to be an efficient m...
-
-
Reaction of FeCl2 with the chelating bis...
Mesoporous poly(triphenylphosphine) with...
Representative tertiary amines were link...
Phenylphosphinic acid and dialkylsulfoxi...
A reduction of various aryl, alkyl, and ...
Triphenylborane (BPh3) was found to cata...
The title reaction was utilized for effi...
Herein, we report a straightforward prot...
-
Base-catalyzed hydrosilylation of nitril...
Azo-functionalized MOPs (Azo-MOPs) were ...
A selective and efficient route for the ...
Aryl Grignard reagents react with N,N-di...
A practical procedure for production of ...
Primary and secondary amines are quickly...
A family of N-methylated and N,N-dimethy...
-
The course of the reaction between N-met...
A simple transition metal-free procedure...
The addition of tert-butyl borate or eth...
Pyrolysis of dimethylnitramine (DMNA) an...
Herein we report a highly meta-selective...
Pt and MoOx co-loaded TiO2 is found to b...
N,N-Diethylaniline·borane (DEANB), a the...
Sodium borohydride and a series of relat...
A mild and highly effective H2O2 oxidati...
-
-
The reaction of IrI[C6H2(CH2NMe 2)-2-R1-...
Amines (primary and secondary) were meth...
A novel method for the preparation of ol...
A library of over 20 cycloplatinated com...
-
Dialkyl-methyleneiminium salts react wit...
Formic acid is used as the sole carbon a...
The chemoselective conversion of a speci...
Transformation of relatively less reacti...
A terminal [Ni-OH] complex1, supported b...
2-chloro-benzaldehyde

dimethyl amine

N,N'-dimethylbenzylamine

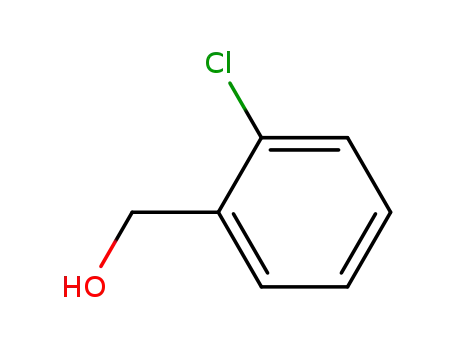
2-Chlorobenzyl alcohol

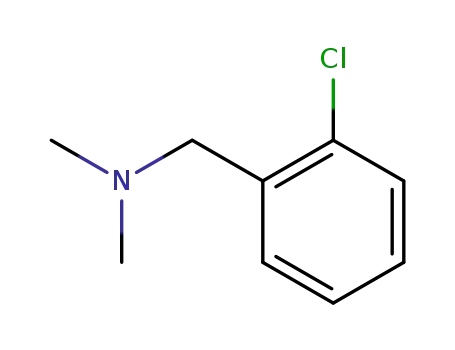
1-(2-chlorophenyl)-N,N-dimethylmethanamine
| Conditions | Yield |
|---|---|
|
With acetic acid; In water; at 99.84 ℃; for 3h; under 3750.38 Torr; Inert atmosphere;
|
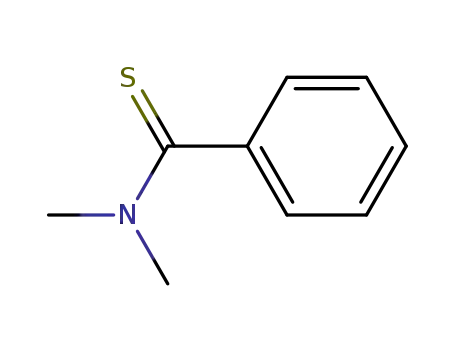
N,N-dimethylthiobenzamide


N,N'-dimethylbenzylamine

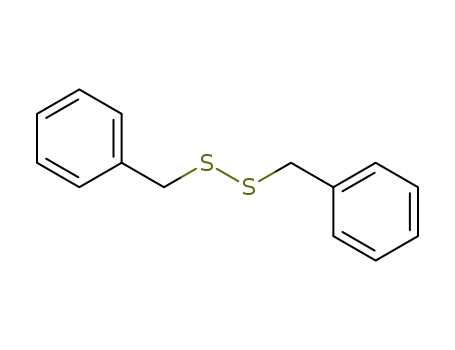
dibenzyl disulphide

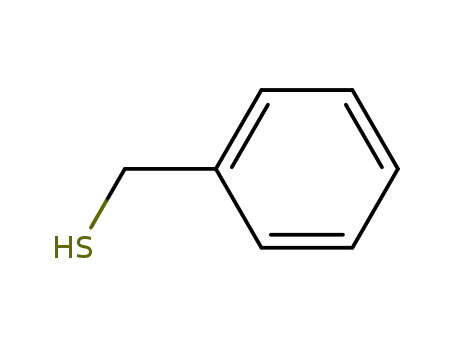
phenylmethanethiol
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In acetonitrile; for 26h; Heating;
|
6% 23% 63% |
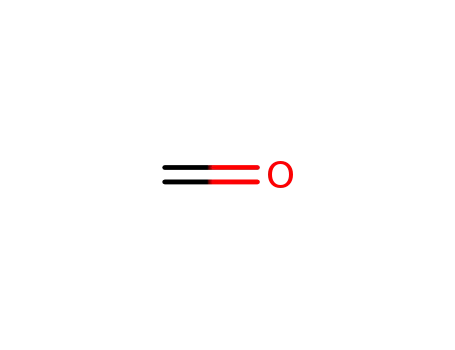
formaldehyd
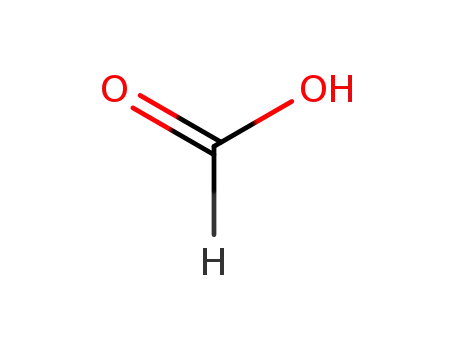
formic acid
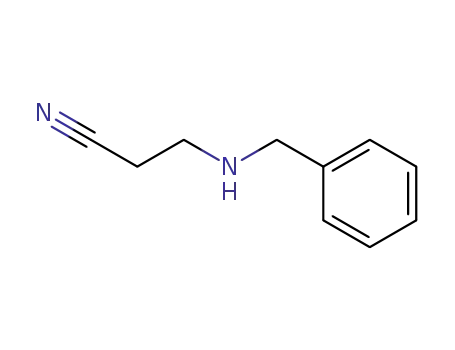
3-(benzylamino)propionitrile
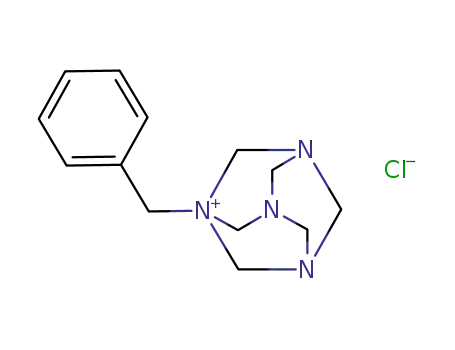
1-benzyl-3,5,7-triaza-1-azoniatricyclo<3,3,1,13,7>decane chloride
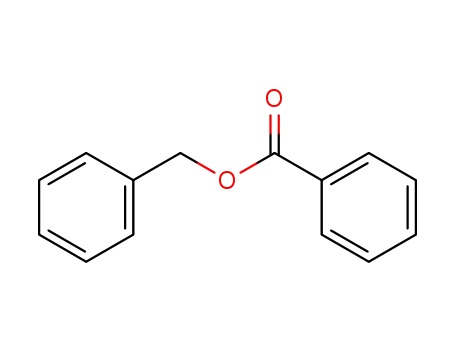
benzoic acid benzyl ester
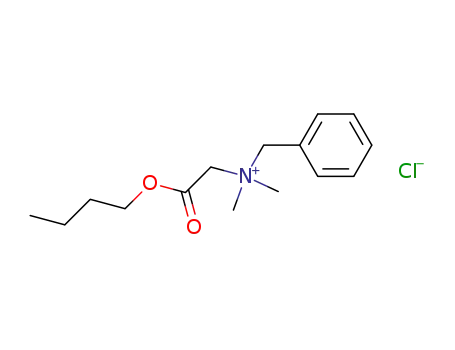
benzyl-butoxycarbonylmethyl-dimethyl-ammonium; chloride
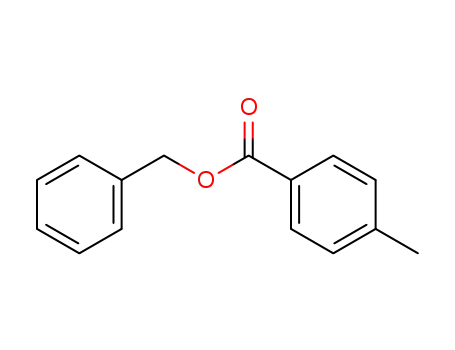
4-methylbenzoic acid benzyl ester
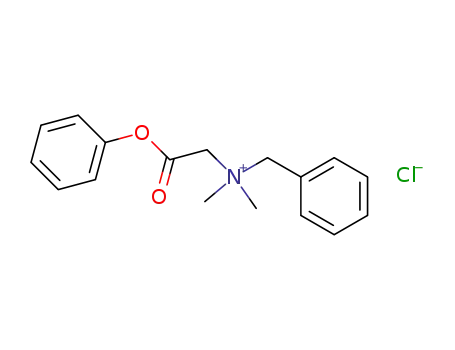
benzyl-dimethyl-phenoxycarbonylmethyl-ammonium; chloride
CAS:80-70-6
CAS:10061-68-4
CAS:1116-76-3
CAS:2212-32-0