Your Location:Home >Products >Inorganic chemicals >Functional compounds >Reagent >10450-60-9
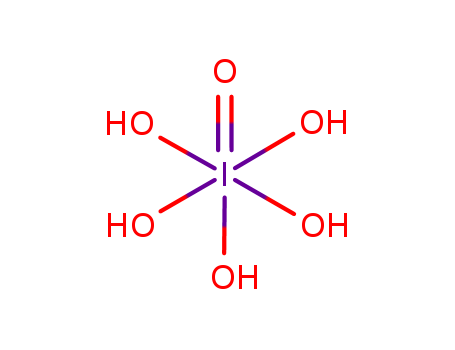

Product Details
| Product Name | Periodic Acid |
| Alias | |
| English | |
| Molecular formula | H5IO6 |
| Molecular weight | 227.94 |
| CAS No | 10450-60-9 |
| EINECS | 233-937-0 |
| Specification | Spec.: AR Appearance Colorless or white crystal Assay(HIO4•2H2O) 99.0%min Clarity test ≤3 Water-insoluble ≤0.01% Ignition residue(SO4) ≤0.05% Iodide ≤0.001% Other halogen(Cl) ≤0.01% |
| Appearance traits | Colorless or white crystal, odorless.It has the deliquescence. |
| Use | A reagent for the determination of carbohydrates by thin layer chromatography. A reagent for the determination of phenylhydrazine by spectrophotometry. |
| Package | 1kg/bottle, 25kg/drum |
| Other product information |
|
General Description |
Periodic(VII) acid, also known as Perjodic acid, is a strong and unstable oxidizing agent with the formula H5IO6. It can be prepared by the reaction of iodine pentoxide with water, and it is one of the most powerful oxides of iodine. Periodic(VII) acid is a colorless, hygroscopic solid with strong oxidizing properties. It is commonly used as a reagent for the oxidation of various organic compounds and as a precursor to other iodine compounds. However, due to its instability, it must be handled with extreme caution, as it can decompose explosively under certain conditions. |
InChI:InChI:1S/H5IO6/c2-1(3,4,5,6)7/h(H5,2,3,4,5,6,7)
Since its inception, the company has continuously expanded its business varieties and fields, to realize the business pattern with the expansion upstream and downstream as well as the complementary of internal and foreign trade. Based on technological innovation and guided by market orientation, we will accelerate the main business strategy, optimize supply chain elements, and build business flow, logistics flow, information flow, and capital flow in one mode, laying a solid foundation for supplying more high-quality and efficient services to a wider range of customers.
Preparative synthesis techniques have be...
iodic acid

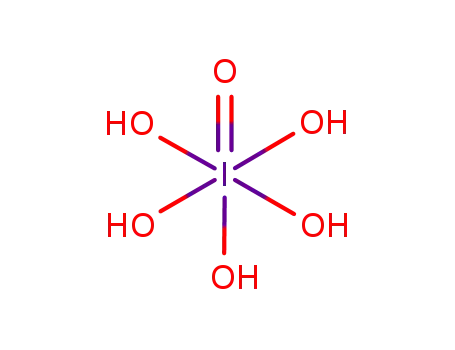
periodic acid
| Conditions | Yield |
|---|---|
|
In water; Electrolysis; special apparatus with Pt wire net covered with PbO2 as anode and current intensity of 12 - 15A; 24h;; evapn. of anode soln. in vac. at 40 - 60°C not to dryness, addn. of some HNO3, further evapn.; crystn.; decantation of mother lye; dissolving H5IO6 in hot concd. HNO3 soln., filtration, recrystn., heating in desiccator over KOH to 40°C;;
|
98% |
|
In water; Electrolysis; special apparatus with Pt wire net covered with PbO2 as anode and current intensity of 12 - 15A; 24h;; evapn. of anode soln. in purified air stream at 70 - 85°C; loss by partial formation of HIO3;;
|
|
|
In sulfuric acid; Electrolysis; 50% HIO3 soln.; anode region: clay cell with MnO2 anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current yield: 0.39%;;
|
|
|
In sulfuric acid; Electrolysis; 50% HIO3 soln.; anode region: clay cell with lead tube covered with PbO2 as anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current density: 0.3 A/cm*cm; current intensity: 2 A; 6 V; 13°C; 4.5 h;; quantitative formation of HIO4 soln. free of IO3(1-); diln., filtration, evapn. on water bath, crystn.; cooling down in desiccator; decantaion of mother lye; recrystn. from water; relative high loss of HIO4 on purification;;
|
|
|
In sulfuric acid; Electrolysis; 50% HIO3 soln.; anode region: clay cell with polished Pt anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current yield: 1.1%;;
|
|
|
In sulfuric acid; aq. H2SO4; Electrolysis; 50% HIO3 soln.; anode region: clay cell with polished Pt anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current yield: 1.1%;;
|
|
|
In sulfuric acid; aq. H2SO4; Electrolysis; 50% HIO3 soln.; anode region: clay cell with MnO2 anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current yield: 0.39%;;
|
|
|
In sulfuric acid; aq. H2SO4; Electrolysis; 50% HIO3 soln.; anode region: clay cell with lead tube covered with PbO2 as anode; cathode region: filled with 2n H2SO4 soln., Pt cathode; current density: 0.3 A/cm*cm; current intensity: 2 A; 6 V; 13°C; 4.5 h;; quantitative formation of HIO4 soln. free of IO3(1-); diln., filtration, evapn. on water bath, crystn.; cooling down in desiccator; decantaion of mother lye; recrystn. from water; relative high loss of HIO4 on purification;;
|
|
|
In water;
|
calcium dimesoperiodate

ammonia

8CaO*3I2O7


periodic acid
| Conditions | Yield |
|---|---|
|
In ammonia; pptn. of a solution of Ca2I2O9 with aq. NH3-solution under formation of product and H5IO6;;
|
|
|
In ammonia; aq. ammonia=NH3; pptn. of a solution of Ca2I2O9 with aq. NH3-solution under formation of product and H5IO6;;
|

ammonia
sodium periodate

iodic acid
nitric acid

4-ethyl-4'-iodobiphenyl
1,3,5-tris(4-iodophenyl)benzene
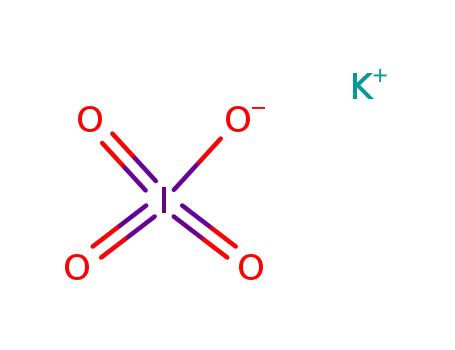
potassium metaperiodate
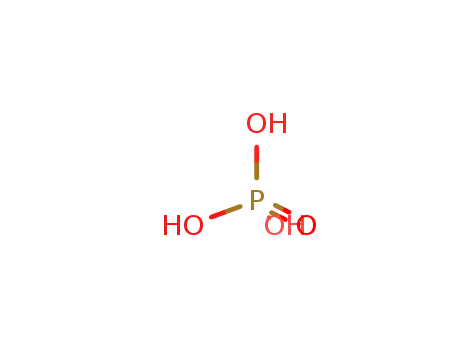
phosphoric acid
CAS:207398-97-8
CAS:12027-06-4
CAS:109-70-6
CAS:110-18-9