Your Location:Home >Products >Inorganic chemicals >Rare light metals(Li,Rb,Cs,Be) >Casium(Cs) >13400-13-0


Product Details
| Product Name | Cesium fluoride |
| Alias | |
| English | |
| Molecular formula | CsF |
| Molecular weight | 191.95 |
| CAS No | 13400-13-0 |
| EINECS | 236-487-3 |
| Specification | 99.0%, 99.5% |
| Appearance traits | Usually in the shape of crystal or white powder, deliquescence, and easily soluble in water, but insoluble in methanol. |
| Use | For the production of fluorinated isocyanate, used as an analytical reagent, also used in the manufacture of optical crystals. Cesium fluoride is a good base in organic chemistry with the high efficiency of Knoevenagel condensation reaction. Cesium fluoride is suitable for the removal of silicon containing protective groups, which can convert many organic silicon compounds into organic silicon fluorine compounds and a carbon negative ion. |
| Package | 1KG/ plastic bottle, 25KG/ cardboard drum |
| Other product information |
|
Hazard |
A poison. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
A poison. Incompatible with benzenediazonium tetrafluoroborate and difluoroamine. When heated to decomposition it emits toxic fumes of F-. |
|
Purification Methods |
Crystallise it from aqueous solution by adding ethanol. |
|
General Description |
Cesium fluoride is an inorganic compound known to be a source of fluoride ion and a catalyst in organic synthesis. It has been used in many organic reactions like 1,4?elimination, desilylation, transesterification, acylation, nucleophilic aromatic substitution, etherification, cross?coupling reactions and so on. |
InChI:InChI=1/Cs.FH/h;1H/q+1;/p-1
Since its inception, the company has continuously expanded its business varieties and fields, to realize the business pattern with the expansion upstream and downstream as well as the complementary of internal and foreign trade. Based on technological innovation and guided by market orientation, we will accelerate the main business strategy, optimize supply chain elements, and build business flow, logistics flow, information flow, and capital flow in one mode, laying a solid foundation for supplying the more high-quality and efficient services to a wider range of customers.
A systematic study of the systems RbF-Na...
For a series of new Mn(III) fluorides th...
Xenon oxide tetrafluoride bears a strong...
Title full: On the crystal structure of ...
The facile axial ligand exchange propert...
The thermolysis of fluorozirconates (M2Z...
[Figure not available: see fulltext.] Cy...
Synthesis of perfluoroethyl isopropyl ke...
The XeOF3- anion has been synthesized as...
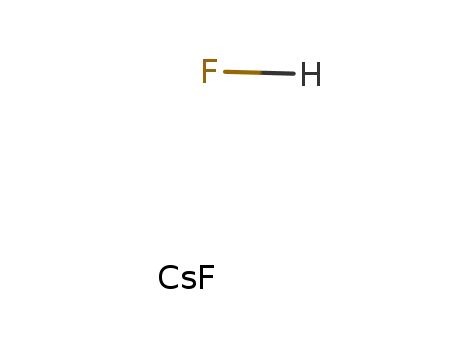
cesium hydrogen fluoride


cesium fluoride
| Conditions | Yield |
|---|---|
|
caesium hydrogen fluoride was heated with NH4F at temp. near the melting point forming CsF;;
|
|
|
caesium hydrogen fluoride was heated with NH4F at temp. near the melting point forming CsF;;
|
|
|
In neat (no solvent); byproducts: HF; CsF*HF decomposed on heating at dark red heat with formation of CsF and HF;;
|
|
|
at 320 - 350 ℃; Inert atmosphere;
|
|
|
|
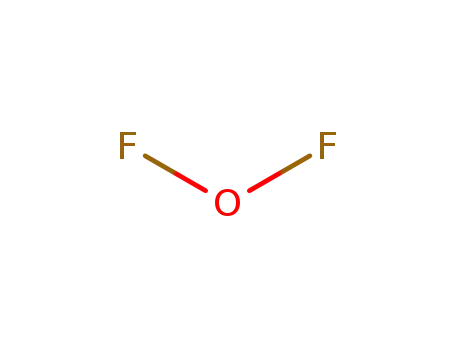
difluoroether

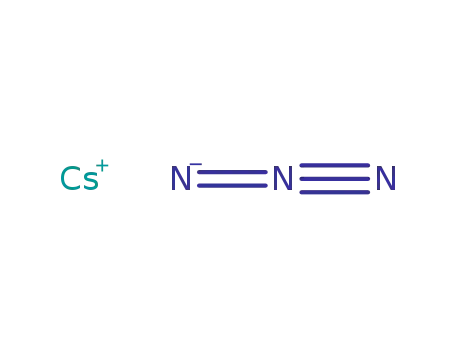
cesium azide


nitrogen


cesium fluoride

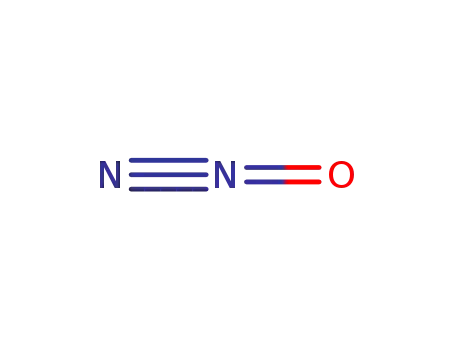
dinitrogen monoxide
| Conditions | Yield |
|---|---|
|
In neat (no solvent); stainless steel autoclave; condensing OF2 onto 3 equiv. CsN3 at -196°C, warming to room temp., heating to 250°C for 15 min; cooling to -196°C, pumping off products; identification by gas-discharge colors, IR spectroscopy and F-sensitive potentiometry;
|

difluoroether
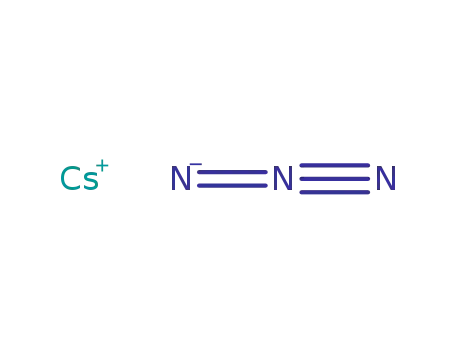
cesium azide

cesium chloride
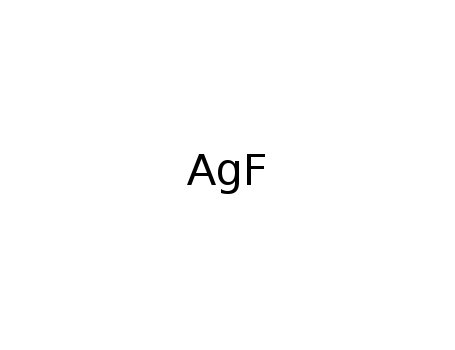
silver fluoride
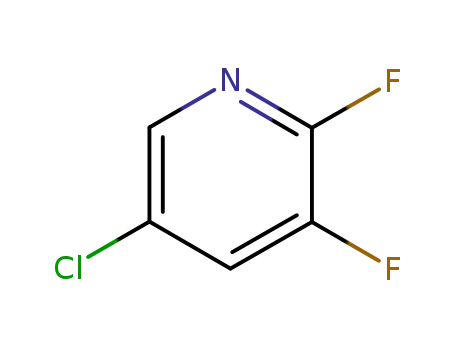
5-chloro-2,3-difluoropyridine

caesium bromide

cesium chloride
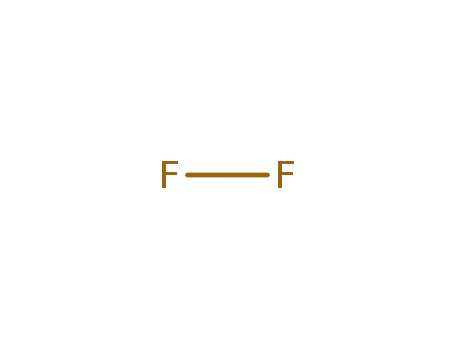
fluorine
CAS:80-70-6
CAS:10061-68-4
CAS:1343-98-2
CAS:35086-59-0