Your Location:Home >Products >Inorganic chemicals >Rare light metals(Li,Rb,Cs,Be) >Rubidium(Rb) >13126-12-0
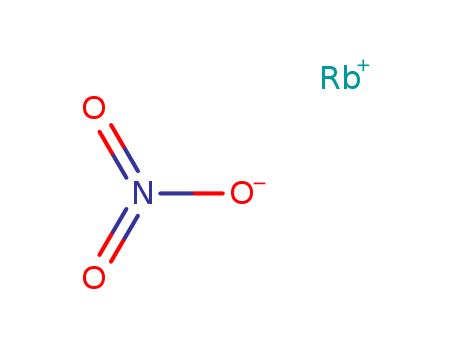

Product Details
| Product Name | Rubidium nitrate |
| Alias | |
| English | |
| Molecular formula | RbNO3 |
| Molecular weight | 147.47 |
| CAS No | 13126-12-0 |
| EINECS | 236-060-1 |
| Specification | 99% 99.5% 99.9% |
| Appearance traits | White crystal. Density (g/mL, 25 C): 3.11. The melting point (S. C): 316. Refractive index: 1.52. Easily soluble in water, soluble in nitric acid, slightly soluble in acetone. Can form adducts with nitric acid. |
| Use | For MHD power generation and preparation of other rubidium salt, also used in catalysts, special ceramics, missile propulsion and containing rubidium single crystal of raw materials and other fields |
| Package | 1 kg / plastic bottle, 25KG/ cardboard drum |
| Other product information |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the nitrate from hot water (0.25mL/g) by cooling to room temperature. |
InChI:InChI=1/NO3.Rb/c2-1(3)4;/q-1;+1
Companies adhering to the "science and technology aim, quality first, customer first, honest and trustworthy" business philosophy, in line with the "pragmatic, integrity, unity, innovation" spirit of enterprise, forge ahead, constantly pursuing higher levels of product quality and service.
The interaction between uranyl nitrate h...
The cubic and monoclinic RbLa1-xEuxP4O12...
Rubidium fluorophosphatozirconates (RFPZ...
Phase equilibria and critical phenomena ...
rubidium carbonate

nitric acid

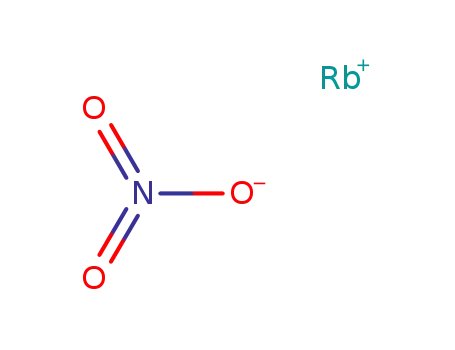
rubidium nitrate
| Conditions | Yield |
|---|---|
|
In nitric acid; react. of Rb2CO3 with the calcd. amount of dild. HNO3;; evapn. to dryness; heating at m.p.; addn. of H2O; evapn. to dryness; drying at 110°C for 3 h;;
|
|
|
In nitric acid; aq. HNO3; react. of Rb2CO3 with the calcd. amount of dild. HNO3;; evapn. to dryness; heating at m.p.; addn. of H2O; evapn. to dryness; drying at 110°C for 3 h;;
|
|
|
at 120 ℃;
|
Rb(1+)*{B(NO3)4}(1-)=Rb{B(NO3)4}

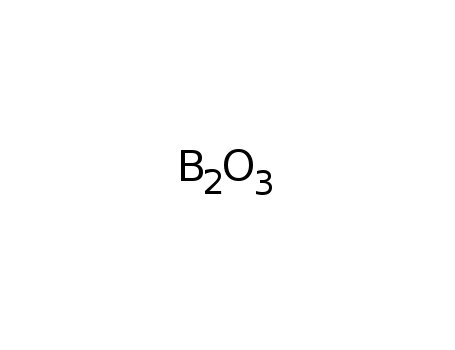
boron trioxide


rubidium nitrate
| Conditions | Yield |
|---|---|
|
by heating;
|
|
|
With alk. or NaOH; In not given; byproducts: NaNO2, NaNO3, O2; producing H2O also;
|

nitric acid

rubidium chloride
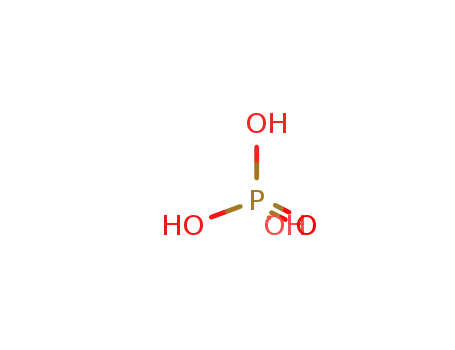
phosphoric acid
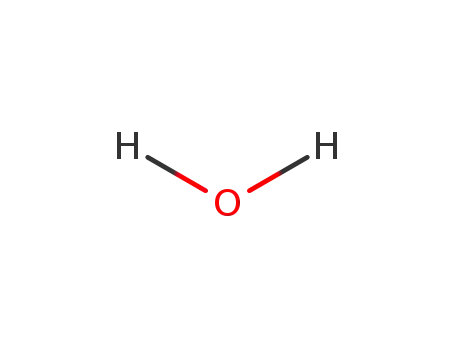
water

rubidium chloride
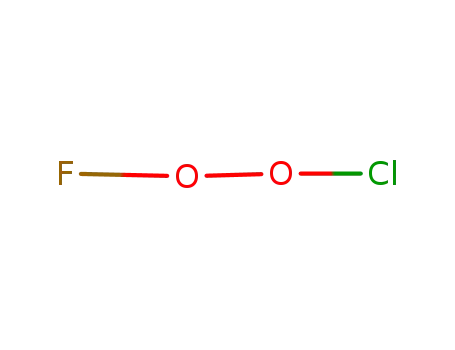
FClO2
nitrylfluoride
rubidium metavanadate
CAS:584-09-8
CAS:7791-11-9
CAS:1704-62-7
CAS:7790-86-5