Your Location:Home >Products >Inorganic chemicals >Rare light metals(Li,Rb,Cs,Be) >Lithium(Li) >7550-35-8


Product Details
| Product Name | Lithium bromide |
| Alias | |
| English | |
| Molecular formula | BrLi |
| Molecular weight | 86.85 |
| CAS No | 7550-35-8 |
| EINECS | 231-439-8 |
| Specification | 99% 99.9% |
| Appearance traits | White cube crystal or granular powder. Stable, in the atmosphere will not break down , volatile and metamorphism. Deliquescence. Slightly bitter taste. Soluble in ethanol and ethyl ether, slightly soluble in pyridine; soluble in organic solvents such as methanol, acetone and ethylene glycol. Insoluble in liquid bromine, cannot form polybrominate. Has a strong water absorption, and very easy to dissolve in water, can form a series of hydrate |
| Use | Used as water vapor absorbent, air temperature regulator, absorption refrigerant, hydrogen chloride removal agent, sedative, and used for camera, battery, etc.. |
| Package | Drum lined with plastic bags, 25kg/ barrels. |
| Other product information |
|
Chemical Description |
Lithium bromide (LiBr) is a chemical compound consisting of lithium (Li) and bromine (Br). It is known for its hygroscopic properties, meaning it readily absorbs water vapor from the atmosphere. LiBr is widely used across various industries due to its chemical and physical characteristics. |
| Special Properties |
Lithium bromide forms hydrates with 1, 2, 3, and 5 moles of water per formula unit: Monohydrate: Well-defined crystal structures. |
| Uses | Core component in industrial absorption chillers, replacing non-CFC refrigerants. Acts as an absorption refrigerant due to its water vapor absorption capability. |
InChI:InChI=1/BrH.Li/h1H;/q;+1/p-1
Since its inception, the company has continuously expanded its business varieties and fields, to realize the business pattern with the expansion upstream and downstream as well as the complementary of internal and foreign trade. Based on technological innovation and guided by market orientation, we will accelerate the main business strategy, optimize supply chain elements, and build business flow, logistics flow, information flow and capital flow in one mode, lay a solid foundation for supplying the more high-quality and efficient services to a wider range of customers.
In this study, the crystal structures of the di- and trihydrates of lithium chloride, lithium bromide and lithium iodide, and the pentahydrates of lithium chloride and lithium bromide have been determined.
In this study three different types of lithium halides; lithium bromide (LiBr), lithium chloride (LiCl) and lithium fluoride (LiF) were used as additives in the making of polyethersulfone (PES) membranes.
Low-grade thermal energy can be converted to electricity by integrating multi-effect distillation and reverse electrodialysis technologies. The energy conversion efficiency of such a system is strongly influenced by the working solution.
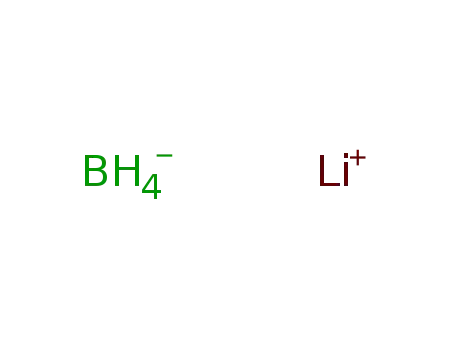
lithium borohydride

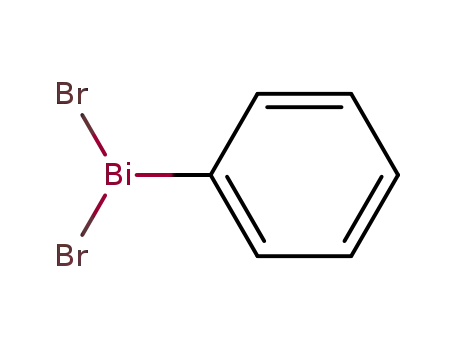
dibromophenylbismuthane

{Bi(C6H5)}x

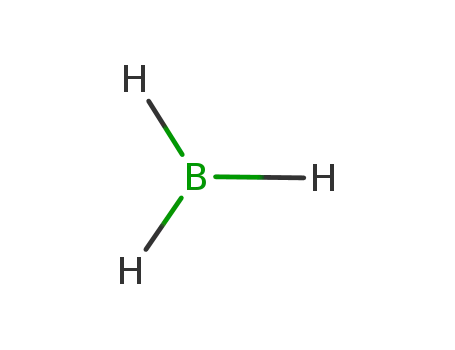
borane


lithium bromide
| Conditions | Yield |
|---|---|
|
With lithium borohydride; In diethyl ether; byproducts: H2; between -70 and -100°C;
|
|
|
With LiBH4; In diethyl ether; byproducts: H2; between -70 and -100°C;
|
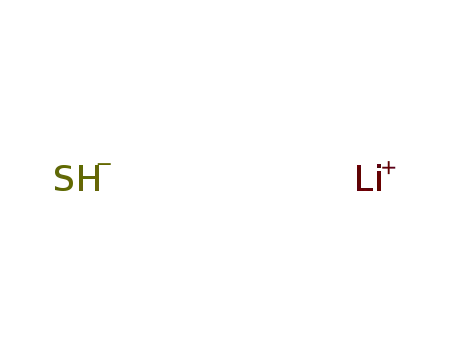
lithium hydrogensulfide

bromine

hydrogen sulfide

sulfur


lithium bromide
| Conditions | Yield |
|---|---|
|
|
bromine
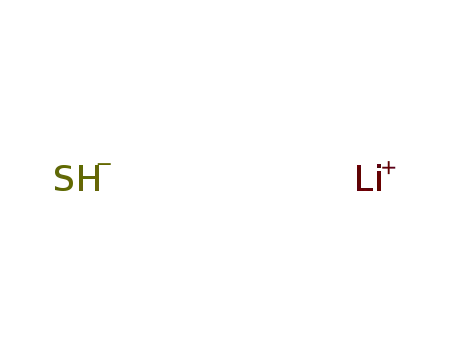
lithium hydrogensulfide
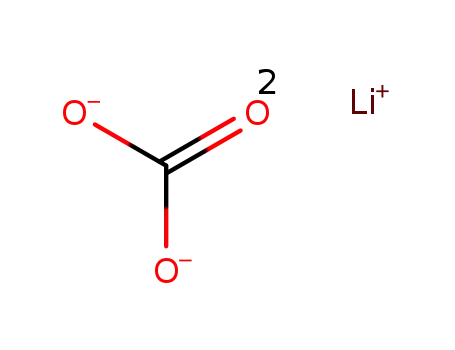
lithium carbonate

lithium
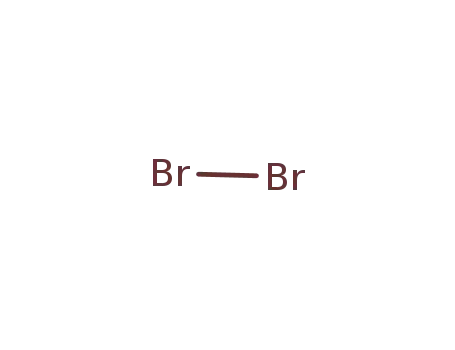
bromine

lithium
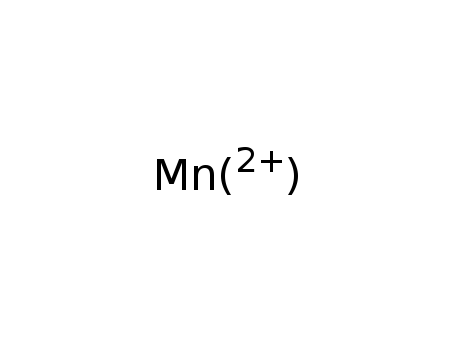
manganese(II)
RuBr2(P(C6H5)3)(C6H5CHO)2
CAS:74-96-4
CAS:109-70-6
CAS:10061-68-4
CAS:7721-01-9