Your Location:Home >Products >Organic Chemicals >Triazole Tetrazole >18039-42-4

Product Details
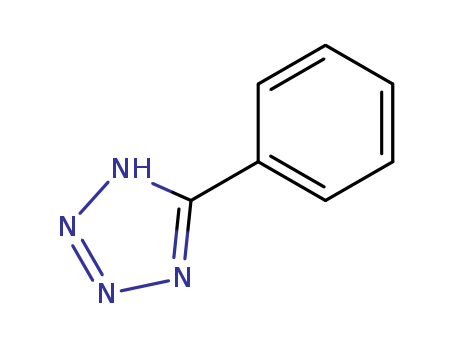
| Product Name | 5-Phenyltetrazole |
| Alias | 5-phenyl-1h-tetrazol |
| English | |
| Molecular formula | C7H6N4 |
| Molecular weight | 146.15 |
| CAS No | 18039-42-4 |
| EINECS | 241-950-8 |
| Specification | Assay: 98%;99.0%. Melting point: 214-216℃ Moisture content:≦0.2% |
| Appearance traits | White crystal powder |
| Use | The intermediates of angiotensinase inhibitors. |
| Package | according to customer's requirement |
| Other product information |
|
Synthesis |
5-Phenyltetrazole can be prepared by reacting benzonitrile with hydrazine or hydrazine salt, and then reacting with nitrous acid. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 80, p. 3908, 1958 DOI: 10.1021/ja01548a028Tetrahedron Letters, 38, p. 1257, 1997 DOI: 10.1016/S0040-4039(97)00052-XTetrahedron, 51, p. 11737, 1995 DOI: 10.1016/0040-4020(95)00725-N |
|
General Description |
Nucleophilic substitution reactions on 1,4-dimethoxybenzene and 1,3,5-trimethoxybenzene by the anions of 5-phenyl-1H-tetrazole has been investigated by paired electrosynthesis. |
InChI:InChI=1/C7H5N4/c1-2-4-6(5-3-1)7-8-10-11-9-7/h1-5H/q-1
Our main markets are China, the United States, Europe, Korea, and Japan. The main business scope includes electronic chemicals, liquid crystal intermediates, high-purity metal compounds, pharmaceutical intermediates, special chemicals, and so on.
Amidoalkylation of 5-aryl(hetaryl)tetraz...
The controlled synthesis of a hierarchic...
Molecules containing tetrazole substitue...
A self-assembled non-covalent metallogel...
-
Novel oxadiazoles bearing 5-phenyl-tetra...
The alkylation of 1-substituted 1H-tetra...
N-Acyloxyalkylation using 5-phenyltetraz...
A novel method for the synthesis of 5-su...
An efficient route to construct 2,5-disu...
A novel method for the one-pot synthesis...
A novel method was developed for the syn...
A simple, green and efficient method has...
A magnetic nanocatalyst was purveyed as ...
A redox catalytic system for oxidation-r...
Today, most synthetic methods are aimed ...
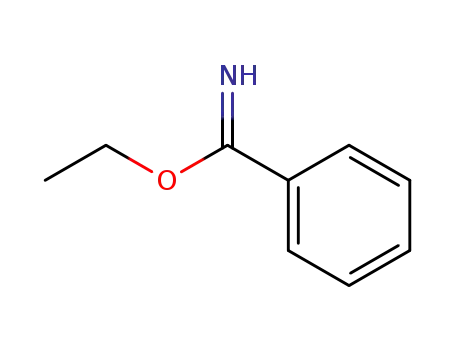
benzimidic acid ethyl ester

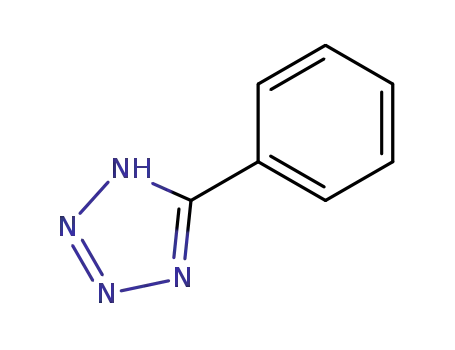
5-Phenyl-1H-tetrazole
| Conditions | Yield |
|---|---|
|
With tris-(2-chloro-ethyl)-amine; benzene;
|
|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate
2: nitric acid; sodium nitrite
With nitric acid; hydrazine hydrate; sodium nitrite;
|
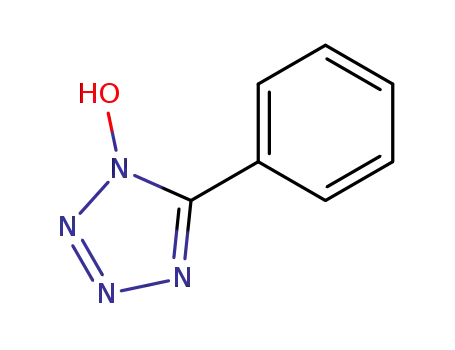
1-hydroxy-5-phenyltetrazole

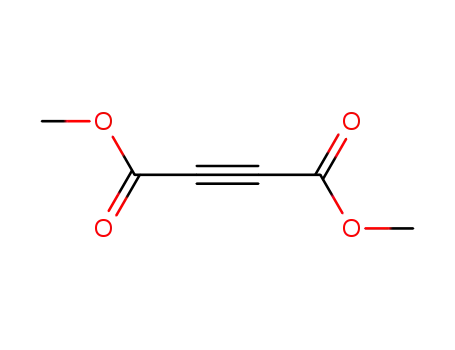
dimethyl acetylenedicarboxylate


5-Phenyl-1H-tetrazole

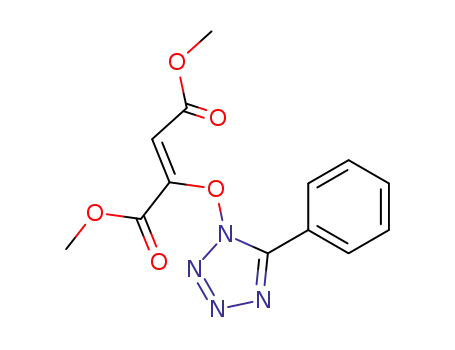
dimethyl 5-phenyl-1-tetrazolyloxy-1,2-ethylenedicarboxylate (Z)

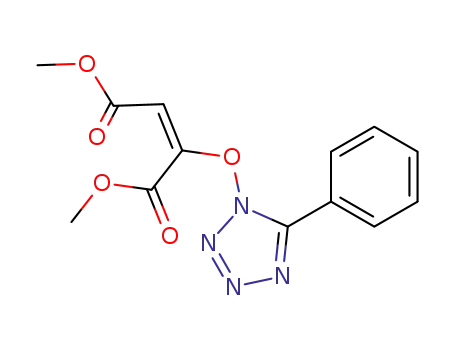
dimethyl 5-phenyl-1-tetrazolyloxy-1,2-ethylenedicarboxylate (E)
| Conditions | Yield |
|---|---|
|
With triethylamine; In methanol; for 18h; Yield given. Yields of byproduct given; Ambient temperature;
|
17% |
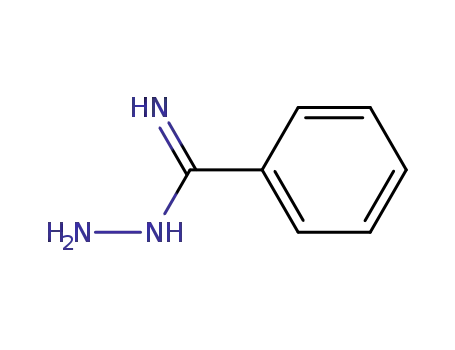
benzamidrazone
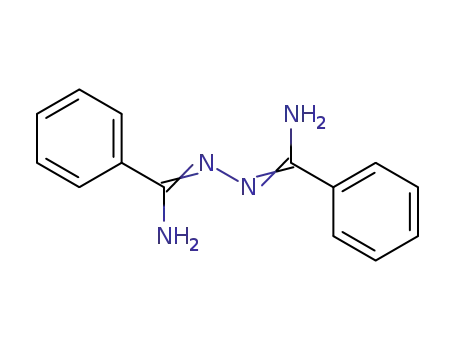
N1-(α-Aminobenzyliden)benzamidrazon

benzonitrile

benzimidic acid ethyl ester
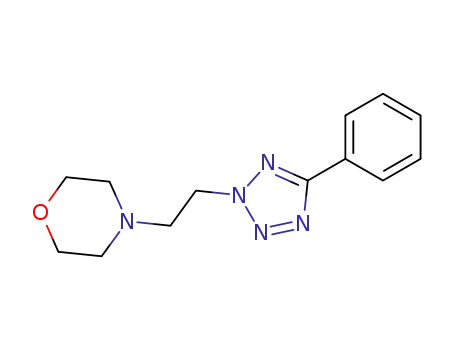
4-[2-(5-phenyl-tetrazol-2-yl)-ethyl]-morpholine
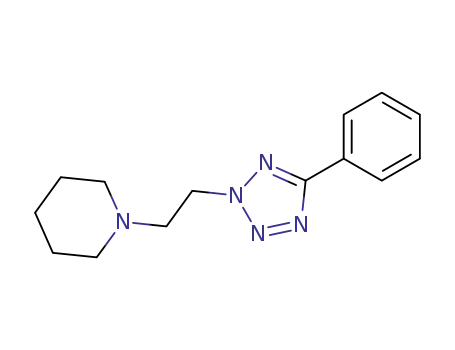
5-phenyl-2-(2-piperidino-ethyl)-2H-tetrazole
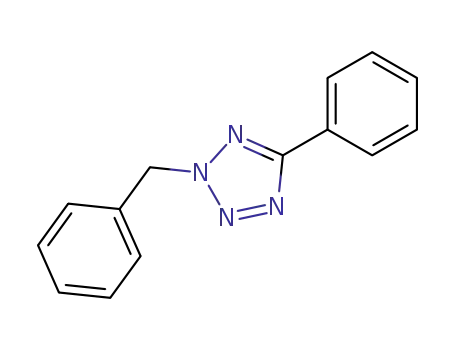
2-benzyl-5-phenyl-2H-tetrazole
1-ethyl-5-diphenyl-1H-1,2,3,4-tetrazole
CAS:80-70-6
CAS:10061-68-4
CAS:68399-81-5
CAS:7365-44-8