Your Location:Home >Products >Organic Chemicals >Crystal intermedates >2628-16-2
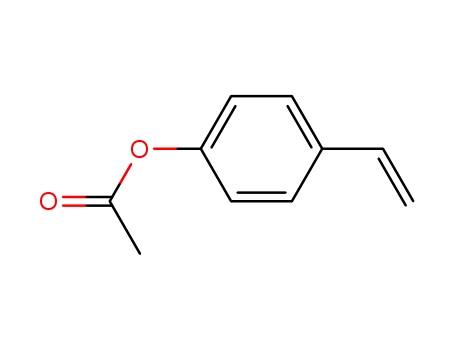
Product Details
| Product Name | 4-Acetoxystyrene |
| Alias | para-acetoxystyrene;4-Ethenylphenyl acetate;4-Vinylphenyl acetate;p-Vinylphenol acetate;p-Acetoxystyrene |
| English | |
| Molecular formula | C10H10O2 |
| Molecular weight | 162.19 |
| CAS No | 2628-16-2 |
| EINECS | |
| Specification | Purity by GC(A%) ≥99.00% Purity by GPC (A%) ≥99.00% Water content ≤0.20% Polymerization Inhibitor ( TBC) 200-300ppm |
| Appearance traits | Colorless liquid |
| Use | Intermediate of Liquid crystal, OLED Materials ,organic intermediate |
| Package | 1kg/bottle, 25kg/drum, or according to customer's require |
| Other product information | Properties: melting point:7-8 °C(lit.) boiling point:260 °C(lit.) density:1.06 g/mL at 25 °C(lit.) flash(ing) point:94°C |
|
Preparation |
The preparation of 4-Acetoxystyrene is as follows:Add p-hydroxystyrene (120g) to a 2L four-neck bottle. triethylamine(106g), phenothiazine (1.2g), Methyl tert-butyl ether (480 g), Dry ice-ethanol bath to -5 to 0 °C, acetyl chloride (86g) was added dropwise with stirring. The internal temperature is controlled at -5 to 0 °C. After the completion of the dropwise addition, the temperature was raised at 10 to 20 °C to continue the reaction for 1 hour. Sampling analysis (central control 1, raw material After completion of the reaction, the mixture was filtered, and the cake was washed with methyl t-butyl ether (50 g × 3). The filtrate was quenched by the addition of 4 g of methanol, and the reaction was stirred for 10 minutes. After completion, phenothiazine (1.2 g) was added and the reaction mixture was concentrated.Methyl tert-butyl ether (580 g) was recovered to give a crude product (160 g).The crude product was distilled under reduced pressure to give the product, p-acetoxy styrene (142 g), yield 87.7%, and sampled for analysis (main content >99%). |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Moderately toxic by ingestion.Slightly toxic by skin contact. An eye irritant. A combustibleliquid. When heated to decomposition it emits acrid smokeand irritating vapors. |
InChI:InChI=1/C10H10O2/c1-3-9-4-6-10(7-5-9)12-8(2)11/h3-7H,1H2,2H3
High-quality chemical talents, abundant product resources, a perfect supply chain, a reasonable organizational structure, and a scientific management model are the core competitiveness of our company's continuous development. Based on good credibility, cooperation, development, and win-win purpose, after years of efforts, the company has established stable cooperative relationships with more than hundreds of chemical enterprises in domestic and established a good reputation and corporate image in the chemical field.
The invention discloses a synthesis meth...
Desulfurization of a variety of thiirane...
An environmentally friendly and efficien...
Both sulfur and fluorine play important ...
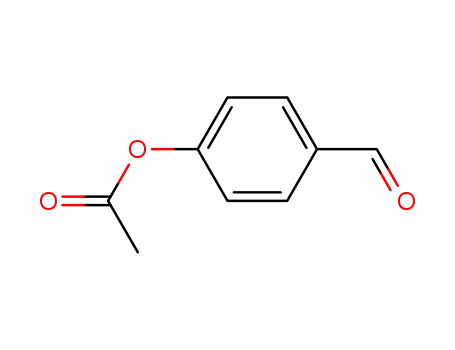
4-formylphenyl acetate

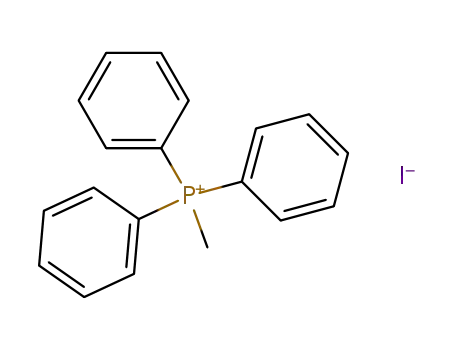
methyl-triphenylphosphonium iodide

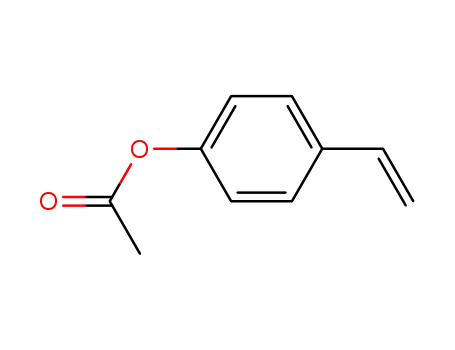
p-acetoxystyrene

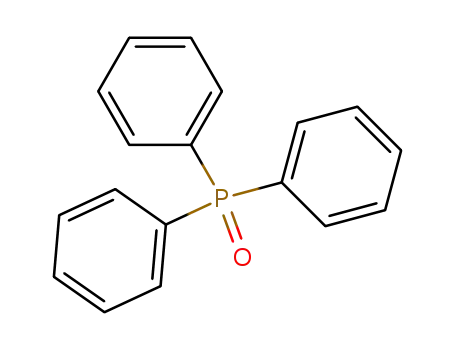
Triphenylphosphine oxide
| Conditions | Yield |
|---|---|
|
With Carbowax 6000 (liquid); potassium carbonate;
|
10% |
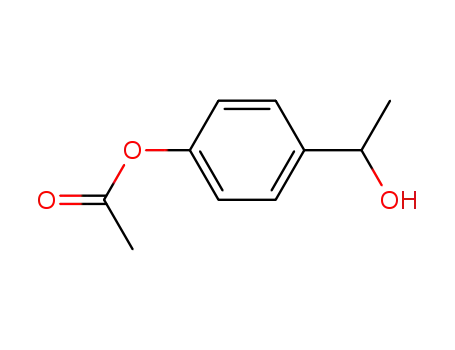
4'-acetoxyphenylmethylcarbinol

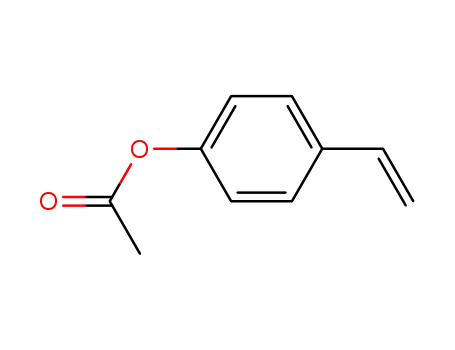
p-acetoxystyrene
| Conditions | Yield |
|---|---|
|
With 10H-phenothiazine; 1-ethyl-3-methylimidazolium hydrogensulfate; at 150 ℃; for 6h; Temperature; Reagent/catalyst;
|
90% |
|
With 4-tert-Butylcatechol; potassium hydroxide; In N,N-dimethyl acetamide; at 100 - 110 ℃; Reagent/catalyst; Temperature;
|
79.3% |
|
With tert-butylcatechol; In toluene; at 90 ℃; for 0.5h; Reagent/catalyst; Temperature; Molecular sieve;
|
78% |
|
With potassium hydrogensulfate; at 175 - 200 ℃;
|
|
|
With acetic anhydride; In N,N-dimethyl-formamide; at 50 ℃;
|
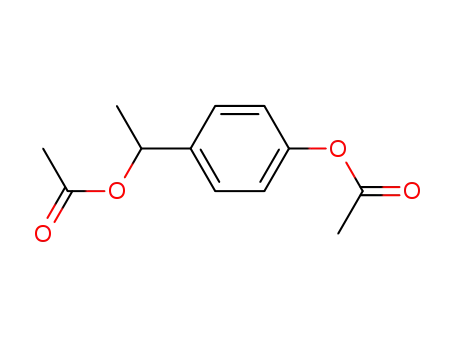
1-acetoxy-4-(1-acetoxy-ethyl)-benzene

4'-acetoxyphenylmethylcarbinol
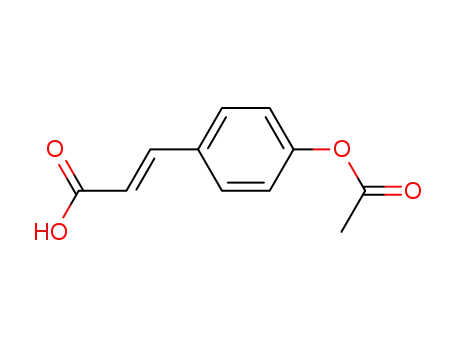
4-acetoxycinnamic acid
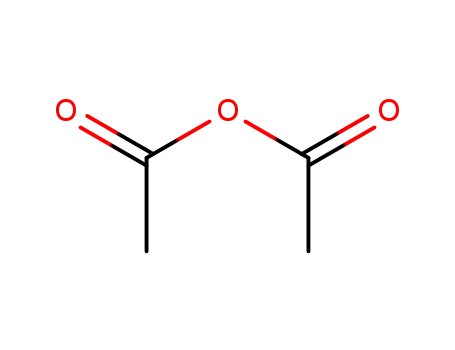
acetic anhydride
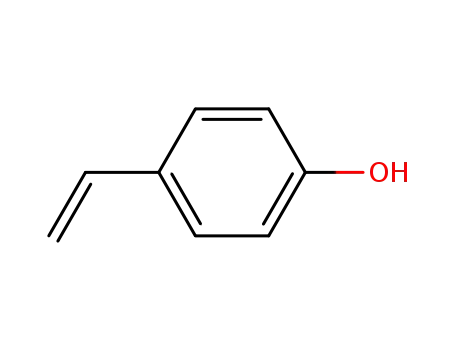
4-Vinylphenol
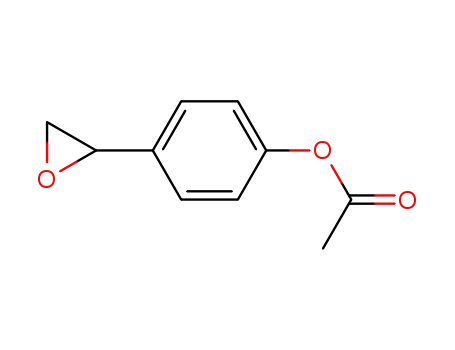
4-(oxiran-2-yl)phenyl acetate
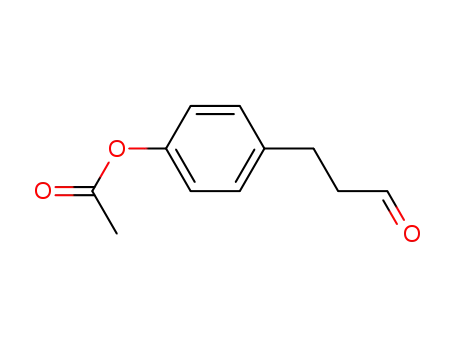
4-(3-oxopropyl)phenyl acetate
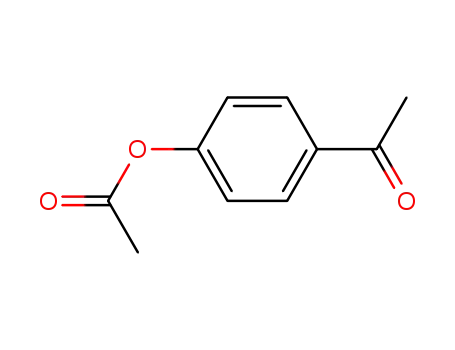
4-acetyloxyacetophenone
CAS:123-00-2
CAS:12182-83-1
CAS:68390-97-6
CAS:288-88-0