Your Location:Home >95418-58-9

Product Details
|
Synthesis |
Preparation of 4-tert-Butoxystyrene (3-tert-Butoxystyrene). To a 2000-mL three-necked round-bottom flask equipped with a dropping funnel, thermometer, reflux condenser, paddle stirrer and nitrogen inlet were placed 19.4 g (0.80 mol) of magnesium turnings and enough freshly dried and distilled tetrahydrofuran (THF) to cover the turnings. There were then added dropwise with stirring a solution of 143.3g (0.78 mol) of freshly distilled 4-bromostyrene (bp 46-47°C (0.03 mm)) in 500mL of THF. After 20mL of the 4-bromostyrene had been added, an exothermic reaction set in and was maintained between 25 and 35°C by adjusting the rate of addition of the bromo compound and with the aid of an ice bath. After the addition had been completed, the reaction mixture was heated to 60°C for 0.5h. Using a NaC1-ice bath, the mixture was cooled to 0°C and a solution of 100.88g (0.52 mol) of tert-butyl peroxybenzoate in 200 mL of THF was added via the dropping funnel at such a rate that the reaction temperature was maintained between 0 and 5°C. After completion of the addition, the reaction mixture was stirred at 25°C for 2 h. The organic layer was separated from the solid magnesium benzoate by decantation and the volume of the solution reduced on a rotary evaporator. The yellow oil that remained was washed with 1000 mL of 3% aqueous HCl solution and the organic layer separated. The aqueous layer was washed with two 200-mL portions of ether, and the ether and organic layers were combined and together washed with two 75-mL portions of a 10% NaOH solution followed by washing with water until the aqueous washings were neutral. After the solution was dried over Na2S04 and the ether was removed via a rotary evaporator, the remaining pale yellow oil was purified by fractional distillation in the presence of a few milligrams of ionol (2,6-ditert-butyl-4-methylphenol) as an inhibitor. There were obtained 46 g (50% yield, bp 45°C (0.02 mm)) of 4-tert-Butoxystyrene having the following elemental analysis. |
InChI:InChI=1/C12H16O/c1-5-10-6-8-11(9-7-10)13-12(2,3)4/h5-9H,1H2,2-4H3
Our main markets are China, the United States, Europe, Korea, and Japan. The main business scope includes electronic chemicals, liquid crystal intermediates, high-purity metal compounds, pharmaceutical intermediates, special chemicals, and so on.
Herein, we report an iron-catalyzed, con...
A nickel(ii)-catalyzed direct olefinatio...
Photochemical decarboxylative cross coup...
A method of performing a chemical reacti...
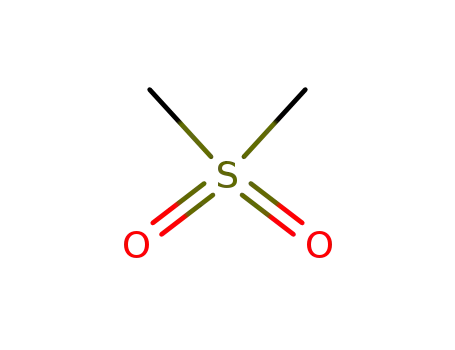
dimethylsulfone


(4-(tert-butoxy)phenyl)methanol


hydrogen

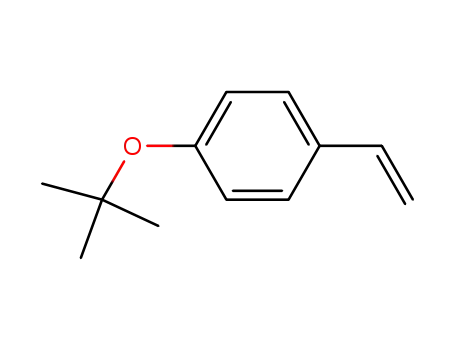
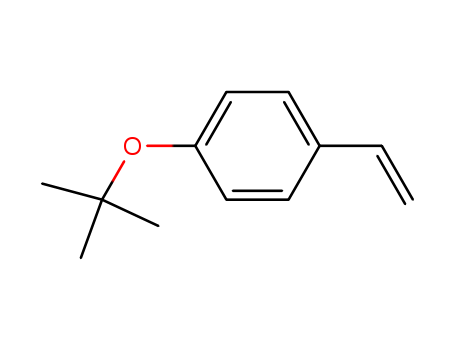
4-tert-butoxystyrene
| Conditions | Yield |
|---|---|
|
With 1,10-Phenanthroline; potassium tert-butylate; iron(II) chloride; In toluene; at 120 ℃; for 8h; Inert atmosphere; Schlenk technique;
|
63% |

vinylboronic acid

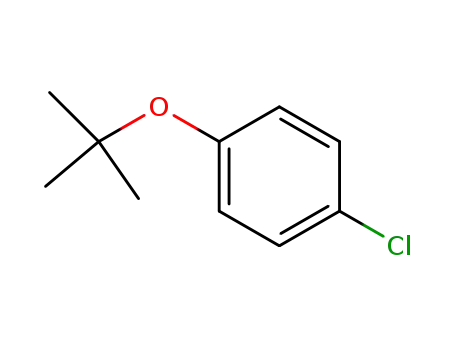
4-tert-butoxychlorobenzene


4-tert-butoxystyrene
| Conditions | Yield |
|---|---|
|
vinylboronic acid; 4-tert-butoxychlorobenzene; With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; In 1,4-dioxane; at 23 ℃; for 0.166667h; Inert atmosphere;
With potassium phosphate; In 1,4-dioxane; water; at 23 - 60 ℃; for 6.5h; Inert atmosphere;
|
79% |
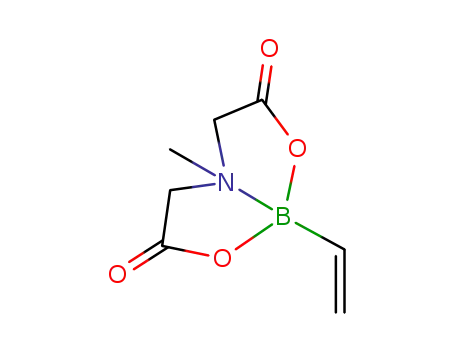
6-methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione

4-tert-butoxychlorobenzene

vinylboronic acid

acrylic acid
CAS:123-00-2
CAS:12182-83-1
CAS:124-28-7
CAS:93164-85-3