Your Location:Home >Products >Organic Chemicals >Polyurethane catalysts >98-94-2

Product Details
| Product Name | N,N-Dimethylcyclohexylamine |
| Alias | Cyclohexyldimethylamine,Dimethylaminocyclohexane |
| English | |
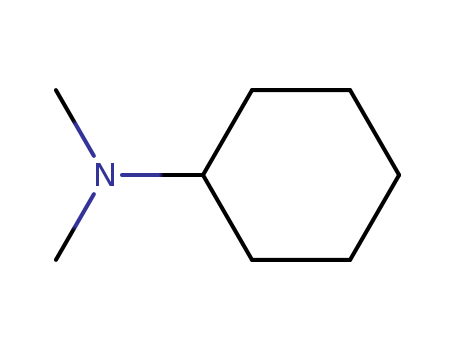
| Molecular formula | C8H17N |
| Molecular weight | 127.23 |
| CAS No | 98-94-2 |
| EINECS | |
| Specification | Specification: Assay(GC):≥99% Water:≤0.5% Color:≤50#(APHA) |
| Appearance traits | Colorless or light yellow transparent liquid. |
| Use | Mainly used for rigid polyurethane catalysts, pharmaceutical intermediates |
| Package | 170kg/drum |
| Other product information | Properties: Melting point:-60 °C Boiling point:160 °C Density:0.849 g/ml at 25 °C(lit.) Refractive index:N20/D 1.454(lit.) Flash(ing) point:108 °F Hazard:UN 2264 8/PG 2 HS code:2921300090 Molecular Structure:
|
|
Definition |
ChEBI: A tertiary amine consisting of cyclohexane having a dimethylamino substituent. |
|
Production Methods |
N,N-Dimethylcyclohexylamine is manufactured either by the reaction of methyl chloride or formaldehyde and hydrogen with cyclohexylamine (HSDB 1989). |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 93, p. 2897, 1971 DOI: 10.1021/ja00741a013Organic Syntheses, Coll. Vol. 6, p. 499, 1988 |
|
General Description |
Colorless liquid with a musky ammonia odor. Less dense than water. |
|
Air & Water Reactions |
Highly flammable. Water soluble. |
|
Reactivity Profile |
N,N-Dimethylcyclohexylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
Inhalation of high concentration of vapor will will produce irritation of the respiratory tract and lungs. Inhalation of large quantities of vapor may be fatal. |
|
Industrial uses |
This amine is used as a catalyst in the production of polyurethane foams. It is also used as an intermediate for rubber accelerators and dyes and in the treatment of textiles. |
|
Safety Profile |
Poison by ingestion. Moderately toxic by inhalation. Whenheated to decomposition it emits toxic fumes of NOx |
|
Metabolism |
There is no record of any metabolic studies with MTV-dime thy ley clohexylamine. However, one can predict that it would be oxidized to the N-oxide by either a cytochrome P-450 system (Damani 1982) or the flavin-containing monooxygenase (Ziegler 1988). Mixed function oxidase enzymes would be expected to produce demethylation (Lindeke and Cho 1982). Many studies describe the metabolism of the parent compound cyclohexylamine (Henderson 1990). |
InChI:InChI=1/C8H17N/c1-9(2)8-6-4-3-5-7-8/h8H,3-7H2,1-2H3
Companies adhering to the "science and technology aim, quality first, customer first, honest and trustworthy" business philosophy, in line with the "pragmatic, integrity, unity, innovation" spirit of enterprise, forge ahead, constantly pursuing higher levels of product quality and service. Warmly welcome customers at home and abroad to establish mutual trust, mutual benefit, and stable cooperation!
Methanol is a potential hydrogen source ...
The invention relates to a preparation m...
The development of heterogeneous, chemos...
The development of Earth-abundant, reusa...
water

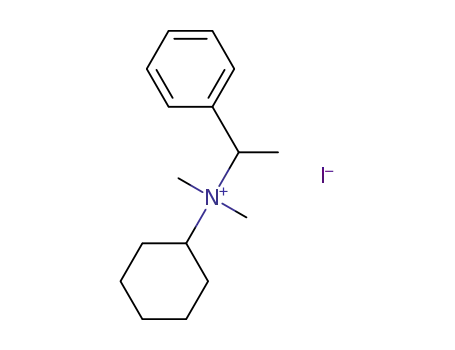
cyclohexyl-dimethyl-(1-phenyl-ethyl)-ammonium; iodide

styrene

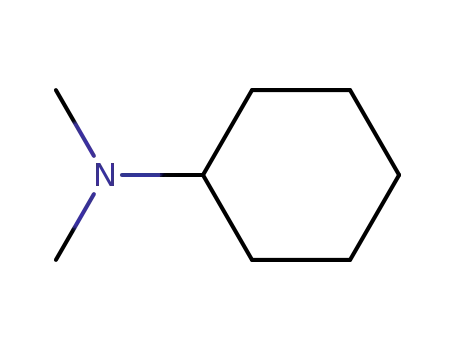
N,N-dimethyl-cyclohexanamine
| Conditions | Yield |
|---|---|
|
die erhaltene freie base gibt beim Erhitzen der waessr.Loesung auf 120grad;
|
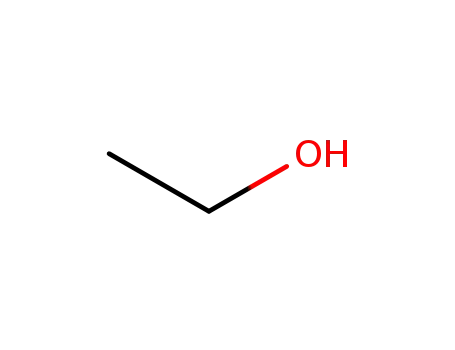
ethanol

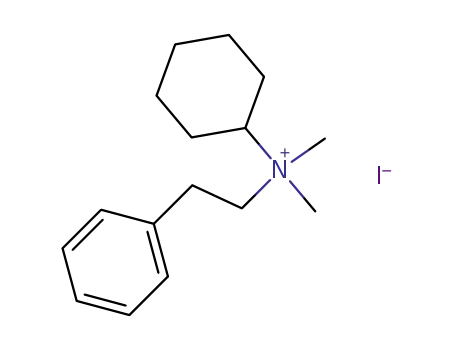
cyclohexyl-dimethyl-phenethyl-ammonium; iodide


styrene


N,N-dimethyl-cyclohexanamine
| Conditions | Yield |
|---|---|
|
bei der thermischen Zersetzung der erhaltenen Base;
|

diazomethane
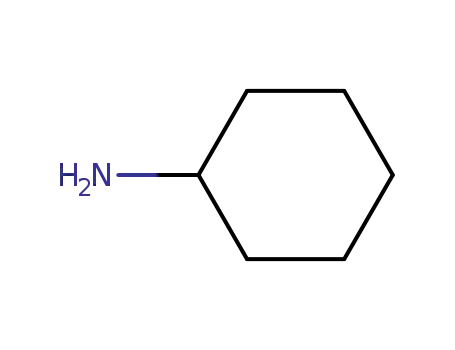
cyclohexylamine
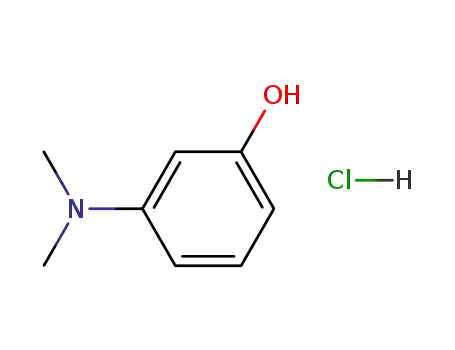
3-dimethylamino-phenol; hydrochloride
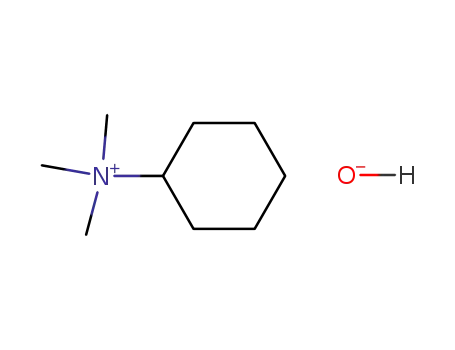
[cyclo-C6H11NMe3][OH]
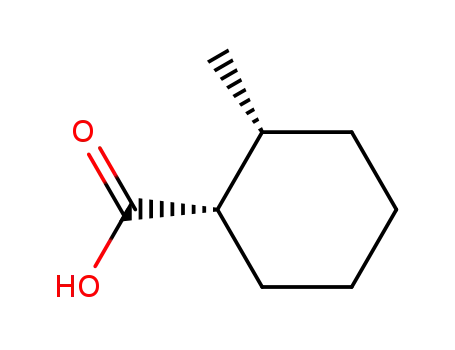
(+-)-cis-2-methyl-cyclohexanecarboxylic acid
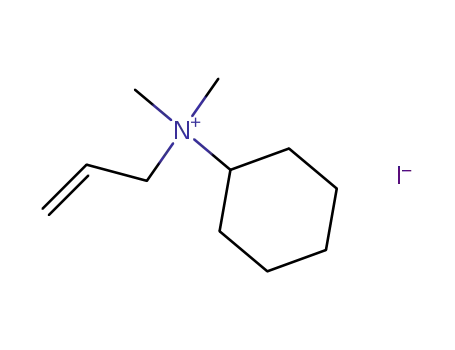
allyl-cyclohexyl-dimethyl-ammonium; iodide
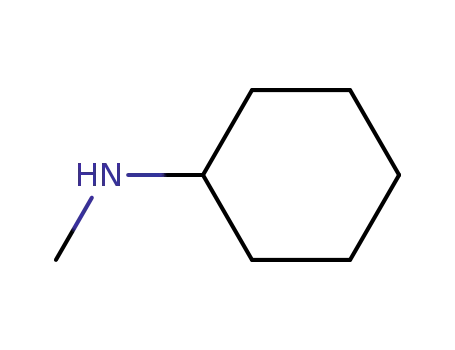
N-methylcyclohexylamine
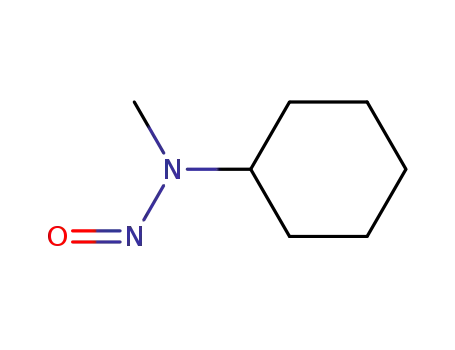
N-nitroso-N-methylcyclohexylamine
CAS:80-70-6
CAS:10061-68-4
CAS: 2687-96-9
CAS:328-50-7