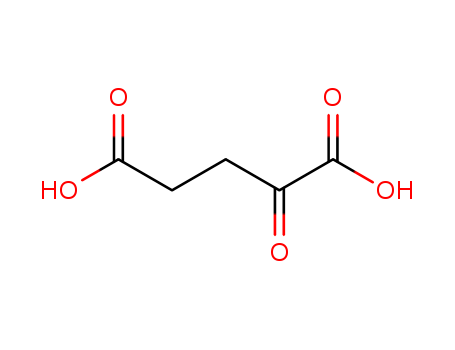

Product Details
|
Preparation |
Synthesis of α-ketoglutaric acid: 225 g of triethyl nitrilotricarboxylate and 600 ml of concentrated hydrochloric acid were mixed and left to pass through. It was concentrated by distillation to 140°C, and the residue was crystallized by cooling to obtain 110-112 g of α-ketoglutaric acid with a yield of 92-93%. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the keto-acid repeatedly from Me2CO/*benzene, EtOAc or ethyl propionate. Dry it in vacuo.[Beilstein 3 IV 1813.] |
|
Definition |
ChEBI: 2-oxoglutaric acid is an oxo dicarboxylic acid that consists of glutaric acid bearing an oxo substituent at position 2. It is an intermediate metabolite in Krebs cycle. It has a role as a fundamental metabolite. It derives from a glutaric acid. It is a conjugate acid of a 2-oxoglutarate(1-). |
|
Application |
2-Ketoglutaric acid is a derivative of glutaric acid. It plays an important role in the metabolism of microbial cells and It acts as precursor for the synthesis of amino acids and nucleotides. α-ketoglutaric acid along with L-arginine can undergo reduction with sodium cyanoborohydride to form the diastereomers, nopaline and isonopaline. α-ketoglutaric acid can be prepared in 1:1 and 2:1 L-arginine alpha-ketoglutarate for sports nutrition. mainly as an ingredient in sports nutrition drinks. |
|
General Description |
2-Ketoglutaric acid is a 2-oxocarboxylic acid (2-OCA), one of the important intermediates in the Krebs cycle, after isocitrate and before succinyl CoA. It is an important nitrogen transporter and is transaminate along with glutamine to form glutamate. It can be used to differentiate microorganisms based on metabolic properties. It also possesses metabolic properties, decreasing the level of hydrogen peroxide in cell culture. |
|
Biotechnological Applications |
a-Ketoglutaric acid is an intermediate of the tricarboxylic acid cycle and the main compound of amino acid and protein metabolism. This organic acid could be used as building-block chemical for the chemical synthesis of heterocycles, dietary supplement, component of infusion solutions, and wound healing compounds (Otto et al. 2013). |
InChI:InChI:1S/C5H6O5/c6-3(5(9)10)1-2-4(7)8/h1-2H2,(H,7,8)(H,9,10)
Our main markets are China, the United States, Europe, Korea, and Japan. The main business scope includes electronic chemicals, liquid crystal intermediates, high-purity metal compounds, pharmaceutical intermediates, special chemicals, and so on.
-
L-Serine biosynthesis, a crucial metabol...
-
-
l-Amino acid deaminases (LAADs; EC 1.4.3...
The alternation of substrate specificity...
Many isocitrate dehydrogenases (IDHs) ar...
Supported metal catalysts are widely use...
Compositions and methods for the product...
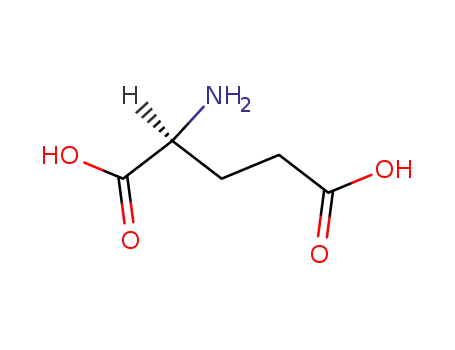
L-glutamic acid

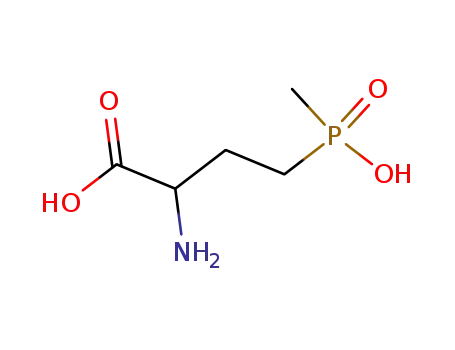
DL-homoalanin-4-yl(methyl)phosphinic acid

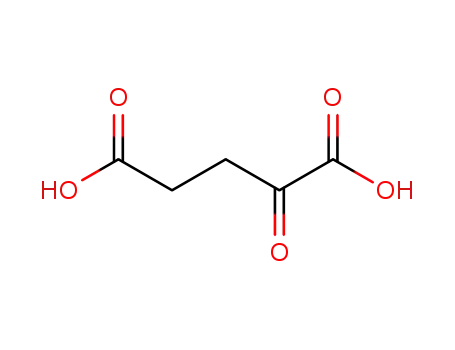
α-ketoglutaric acid

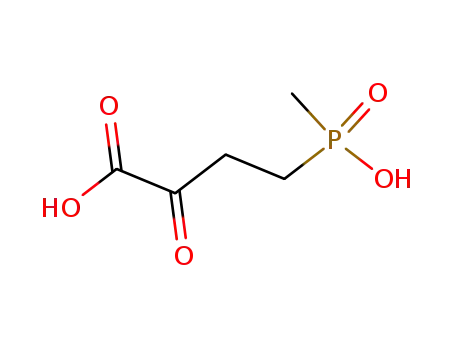
4-(hydroxymethylphosphinyl)-2-oxobutanoic acid

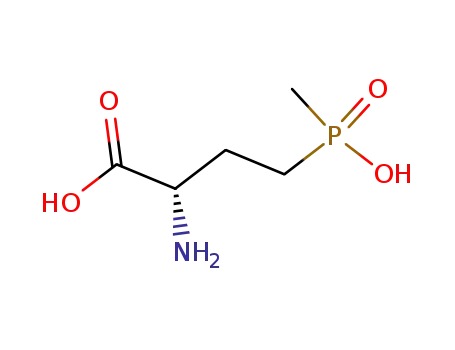
L-phosphinothricin
| Conditions | Yield |
|---|---|
|
With magnesium(II) chloride hexahydrate; pyridoxal 5'-phosphate; E.coli gabT transaminase E211S mutant; thiamine diphosphate; sodium hydroxide; In aq. phosphate buffer; at 30 ℃; for 22.5h; pH=Ca.7.5; Enzymatic reaction;
|
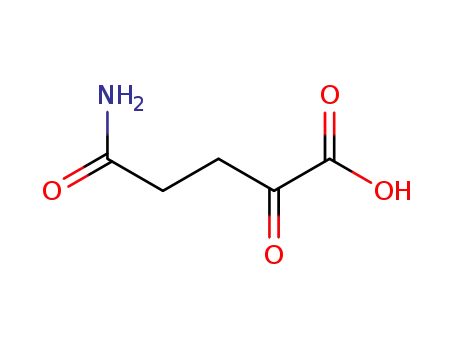
L-glutamine


α-ketoglutaric acid

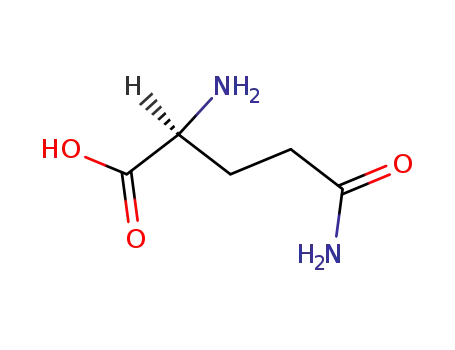
2-keto-glutaramic acid
| Conditions | Yield |
|---|---|
|
enzymatische Reaktion mit 2-Oxo-carbonsaeuren;
|
methanol
1-bromo-cyclopropane-1,2-dicarboxylic acid
1-hydroxy-cyclopropane-1,2-dicarboxylic acid

L-glutamine
2-Oxo-glutarsaeure-isonicotinoylhydrazon
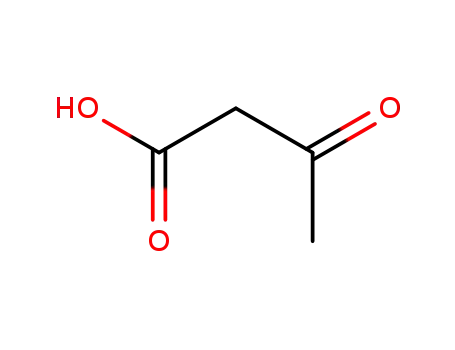
2-acetoacetic acid

L-glutamic acid
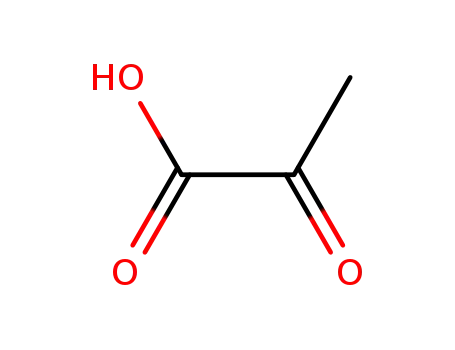
2-oxo-propionic acid
CAS:123-00-2
CAS:12182-83-1
CAS:98-94-2
CAS:96-48-0