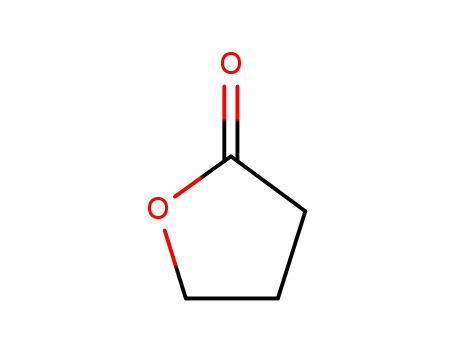

Product Details
|
Chemical properties |
Gamma-Butyrolactone (GBL) is a colorless oily liquid. It is miscible with water and the general organic and slightly soluble in aliphatic hydrocarbons. GBL has been characterized as having an intense bitter taste with faint to pleasant odor. |
|
Production |
Maleic anhydride hydrogenation method is an advanced technology developed in 1970s. It can produce tetrahydrofuran and γ-butyrolactone in any proportion with a hydrogenation reaction, and the usual ratio is tetrachlorofuran: γ-butyrolactone = 3-4:1. There are many production enterprises, but usually in small scale. The average level is 300t/a. The production capacity account for 30% of the total domestic production capacity.? 2.1, 4-butanediol dehydrogenation reactor is a tube array reactor, filled with flake copper catalyst (with zinc oxide as the carrier). The reaction temperature is controlled at 230-240 ° C. The yield of the product is obtained by reduced pressure distillation of and the yield is above 77%. |
|
Category |
Flammable substance |
|
Toxicity grading |
Middle |
|
Acute toxicity |
oral-rat LD50: 1540 mg/kg; oral-mouse LD50: 1720 mg/kg |
|
Hazardous characteristics of the explosive |
Explosible when react with butanol, 2,4-dichlorophenol and sodium hydroxide |
|
Flammability hazard |
Flammable in case of heat, open flame; being able to react with oxidant; releasing toxic pungent smoke when in the process of pyrolysis. |
|
Storage and transportation properties |
Make sure ventilating, low temperature and drying in the warehouse; separate from the oxidant; prevent fires. |
|
Extinguishing agent |
Dry powder, carbon dioxide, foam |
|
Definition |
ChEBI: A butan-4-olide that is tetrahydrofuran substituted by an oxo group at position 2. |
|
Preparation |
γ-Butyrolactone is produced by gas phase 1,4-butanediol under the action of Cu catalyst to produce product γ-butyrolactone and by-product hydrogen. Crude γ-Butyrolactone is purified to remove light and heavy components, with a purity of more than 99.5%, as an export product. At the same time, the by-product hydrogen is sent to other hydrogenation processes for recycling after removing CO and CO2 through the methanation process. |
|
Aroma threshold values |
Detection: 20 to 50 ppm |
|
Taste threshold values |
Taste characteristic at 75 ppm: milky, creamy with fruity peach-like afternotes. |
|
General Description |
Clear colorless oily liquid with a pleasant odor. |
|
Reactivity Profile |
gamma-Butyrolactone can react with oxidizing materials, inorganic acids and bases, alcohols and amines. Rapidly hydrolyzed by bases and slowly hydrolyzed by acids. gamma-Butyrolactone is volatile with steam. . The combination of the lactone, butanol, 2,4-dichlorophenol, and sodium hydroxide in the attempted synthesis of 2,4-dichlorophenoxybutyric acid caused a thermal runaway reaction that eventually exploded, [CISHC Chem. Safety Summ., 1977, 48, 3]. |
|
Fire Hazard |
gamma-Butyrolactone is combustible. |
|
Biochem/physiol Actions |
Precursor of γ-hydroxybutyric acid (GHB). It blocks dopamine release by blocking impulse flow in dopaminergic neurons. Pretreatment with γ-butyrolactone allows detection of autoreceptor-induced dopamine release. |
|
Safety Profile |
Moderately toxic by ingestion, intravenous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data by skin contact. Mutation data reported. Less acutely toxic than ppropiolactone. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, alcohol foam, CO2, dry chemical. Potentially explosive reaction with butanol + 2,4 dichlorophenol + sodium hydroxide. When heated to decomposition it emits acrid and irritating fumes. |
|
Potential Exposure |
Used as a chemical intermediate for making other chemicals, including pesticides, cosmetics, and pharmaceuticals; as a solvent for paint, nail polish removers, and industrial chemicals. Used in electronics, drilling and petroleum industries as a stabilizer and solvent. Used as a flavoring agent in various foods and beverages, including grains and breakfast foods, candy, and alcoholic and nonalcoholic drinks. Drug of abuse: the United States Food and Drug Administration has warned the public not to purchase or consume products, containing gamma-butyrolactone (GBL). FDA has also asked the companies that manufacture these products to voluntarily recall them. The agency has received reports of serious health problems—some that are potentially life-threatening—associated with the use of these products. Although labeled as dietary supplements and marketed under various brand names, these products are illegally marketed unapproved new drugs. False advertising claims include building muscles, improved physical performance, enhanced sex, reduced stress and induced sleep |
|
Shipping |
Listed by some sources as unregulated. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required. |
|
Purification Methods |
Dry the lactone over anhydrous CaSO4, then fractionally distil it. Handle it in a fume cupboard due to its TOXICITY. [Beilstein 17 V 7.] |
|
Incompatibilities |
4-Butyrolactone is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, alcohols, amines, strong and inorganic acids, strong bases. Rapidly hydrolyzed by bases and slowly hydrolyzed by acids. It is hygroscopic and volatile with steam. Combustible; vapor may form explosive mixture with air. |
|
Waste Disposal |
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. All federal, state, and local environmental regulations must be observed. |
InChI:InChI=1/C4H8O4/c1-2(5)3(6)4(7)8/h2-3,5-6H,1H3,(H,7,8)
Hangzhou Ocean Chemical Co., Ltd. is a chemical supplier that provides stable product quality, unique technical support, and high-quality service for global customers, Headquarters is located in Hangzhou with a superior entrepreneurial environment and business climate.
γ-Butyrolactone was produced highly sele...
In this paper, Au/TiO2 nanocomposite was...
Spiropyrans bearing an N-alkylcarboxylat...
-
-
The reactions of alkylperoxyl radicals w...
-
In this study, a novel, strategic method...
With the aim of applying redox-neutral c...
-
The isomerization of 2-butyne-1,4-diol t...
Primary N-C adducts of γ-aminobutyric ac...
Au/TiO2 catalysts prepared by the deposi...
γ-Valerolactone can be synthesized by re...
Cu-CeO2-Al2O3 catalysts were prepared by...
Selenium-doped TiO2 has been used for th...
Optically active δ- and γ-lactones are o...
Heterogeneous palladium catalysts with h...
-
-
Acid catalysed hydrolyses of spiroorthoe...
The efficient hydrogenation of levulinic...
A coupling process of the hydrogenation ...
The two-stage hydrogenation of maleic an...
Cyclobutanones were selectively oxidized...
A series of methacrylates bearing bicycl...
The assembly of heterometallic complexes...
We report the dehydrogenative synthesis ...
Here, we disclose a new copper(i)-Schiff...
Catalytic selective hydrogenation of est...
This study examined a hydrogen transfer ...
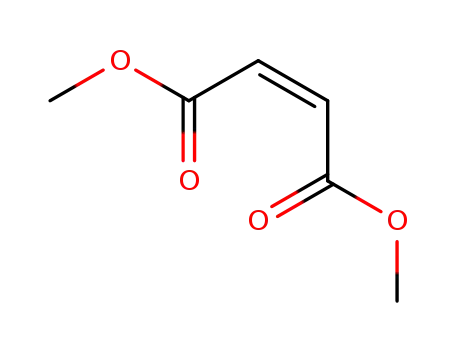
dimethyl cis-but-2-ene-1,4-dioate

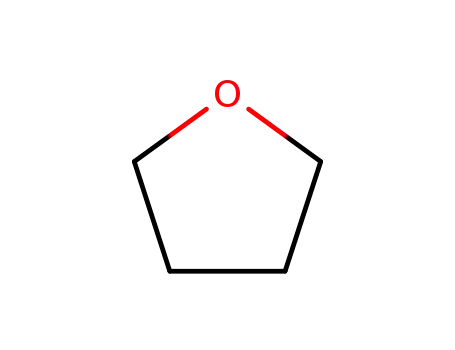
tetrahydrofuran

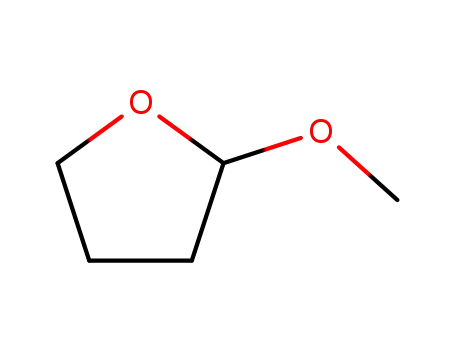
2-methoxytetrahydrofuran

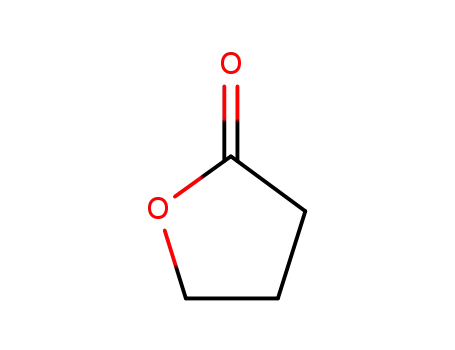
4-butanolide

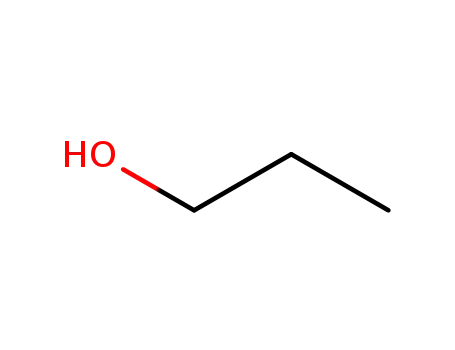
propan-1-ol

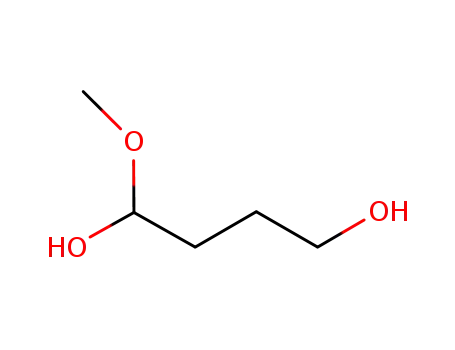
1-methoxy-1,4-butanediol

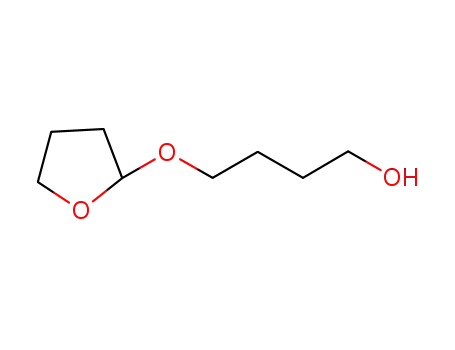
2-(4'-hydroxybutoxy)-tetrahydrofuran

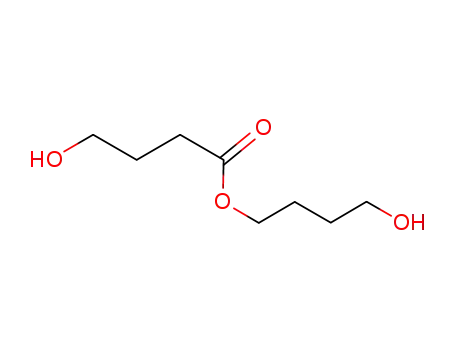
4-hydroxy-butanoic acid 4-hydroxybutyl ester

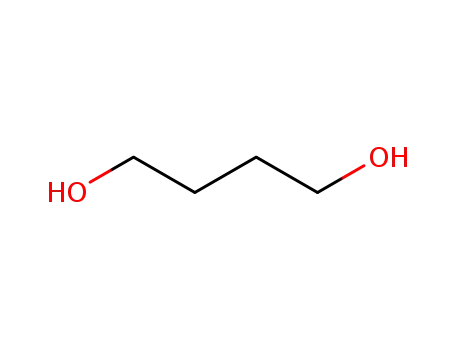
Butane-1,4-diol

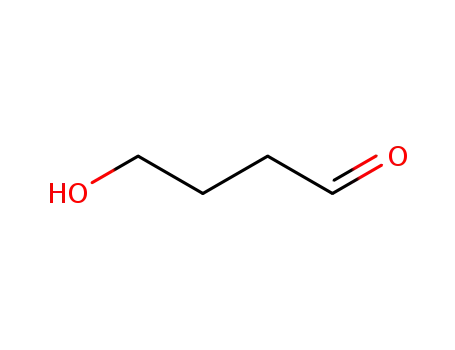
4-hydroxybutyraldehyde

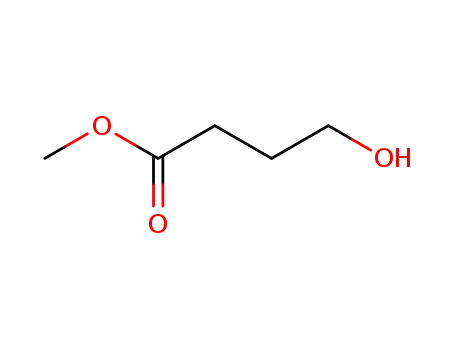
methyl 4-hydroxybutanoate

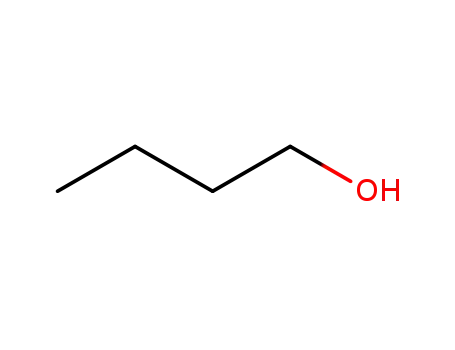
butan-1-ol
| Conditions | Yield |
|---|---|
|
With hydrogen; at 190 ℃; under 46504.7 Torr; Gas phase;
|
79.1% 10.4% 5.3% |

dimethyl cis-but-2-ene-1,4-dioate


tetrahydrofuran


2-methoxytetrahydrofuran


4-butanolide


propan-1-ol


2-(4'-hydroxybutoxy)-tetrahydrofuran


Butane-1,4-diol


butan-1-ol
| Conditions | Yield |
|---|---|
|
With hydrogen; copper catalyst, T 4489, Sud-Chemie AG, Munich; at 150 - 280 ℃; under 187519 Torr; Neat liquid(s) and gas(es)/vapour(s);
|
98% 1% 0.4% 0.5% |

tetrahydrofuran
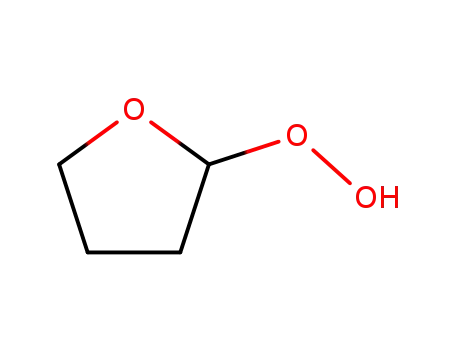
2-hydroperoxytetrahydrofuran
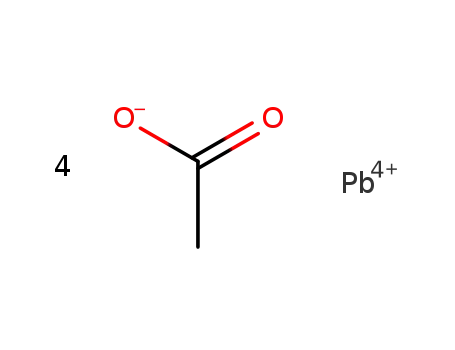
lead(IV) tetraacetate
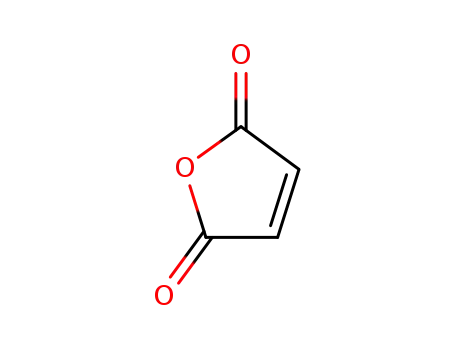
maleic anhydride
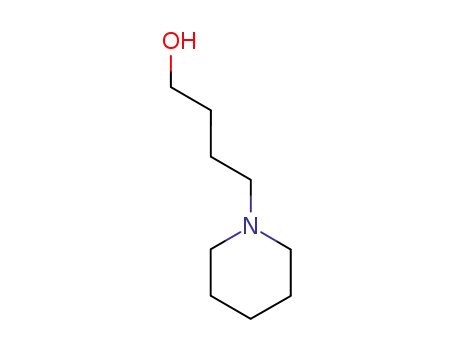
4-piperidin-1-yl-butan-1-ol
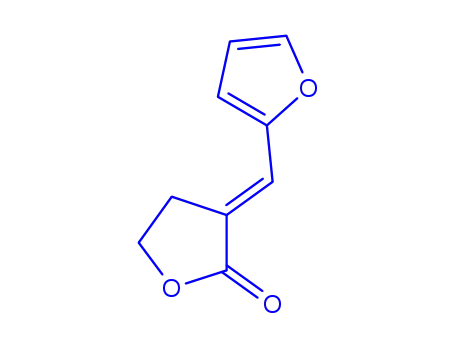
(E)-α-(2-furylmethylene)-γ-butyrolactone
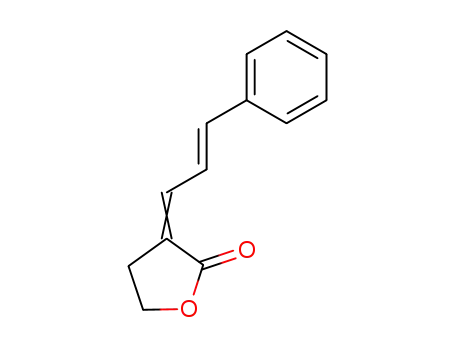
α-(3-phenyl-2-propenylidene)-γ-butyrolactone
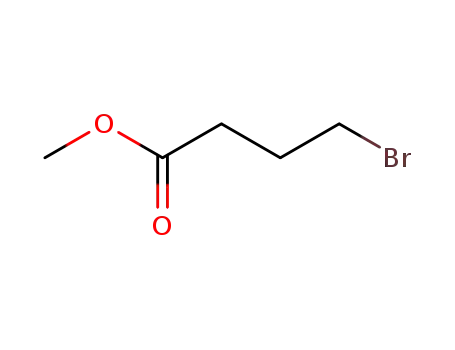
Methyl 4-bromobutyrate
CAS:123-00-2
CAS:12182-83-1
CAS:328-50-7
CAS:643-79-8