Your Location:Home >Products >Organic Chemicals >Cosmetics,Perfume,Others >77-71-4

Product Details
| Product Name | 5,5-Dimethylhydantoin |
| Alias | 2,4-Imidazolidinedione,5,5-dimethyl-; 4-Imidazolidinedione,5,5-dimethyl-2 |
| English | |
| Molecular formula | C5H8N2O2 |
| Molecular weight | 128.13 |
| CAS No | 77-71-4 |
| EINECS | 201-051-3 |
| Specification | Assay ≥99% Melting point: 174~178°C Moisture ≤0.5% PH 4.5-6.0 Ash ≤0.1% |
| Appearance traits | White crystal |
| Use | Used for amino acid synthesis, it can also be used as special epoxy resin and water soluble resin, fungicide, antiseptic and so on. |
| Package | 25KG/fiber drum |
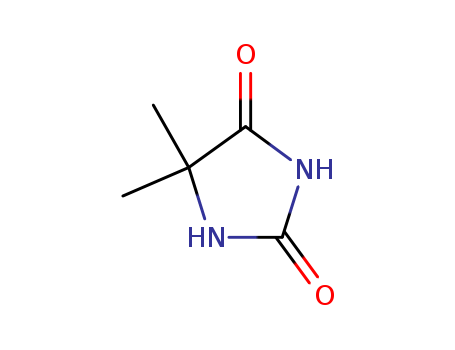
| Other product information | Hazard: None Molecular Structure:
|
|
Purification Methods |
Crystallise the hydantoin from EtOH and sublime it in vacuo. [Beilstein 24 III/IV 1097.] |
|
Uses and Mechanism of Action |
5,5-Dimethylhydantoin(DMH) is utilized in various fields, including antimicrobial finishing of textiles, polymer chemistry, and electrochemistry. It is synthesized for specific applications, such as in textile finishing and polymer functionalization. |
InChI:InChI=1/C5H8N2O2/c1-5(2)3(8)6-4(9)7-5/h1-2H3,(H2,6,7,8,9)
Companies adhering to the "science and technology aim, quality first, customer first, honest and trustworthy" business philosophy, in line with the "pragmatic, integrity, unity, innovation" spirit of enterprise, forge ahead, constantly pursuing higher levels of product quality and service. Warmly welcome customers at home and abroad to establish mutual trust, mutual benefit, and stable cooperation!
-
A continuous Bucherer-Bergs hydantoin sy...
A series of new 3-arylsulfonylimidazolid...
The eco-friendly preparation of 5- and 5...
The invention relates to novel amidoalky...
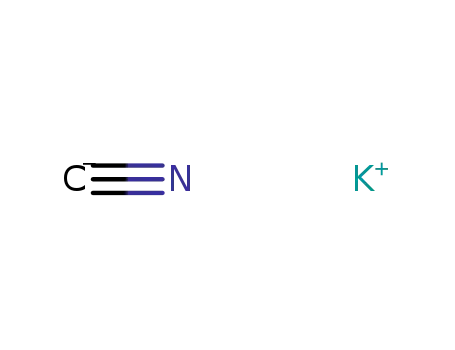
potassium cyanide

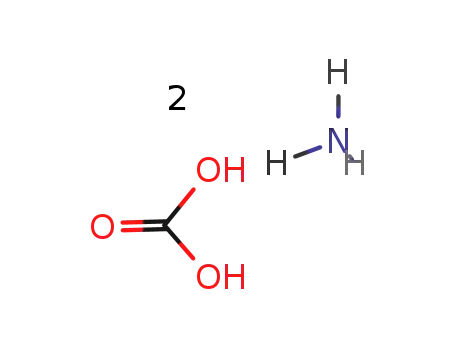
ammonium carbonate

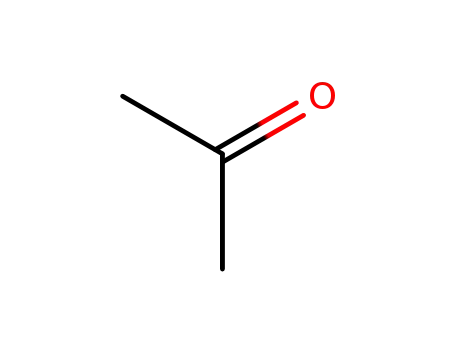
acetone

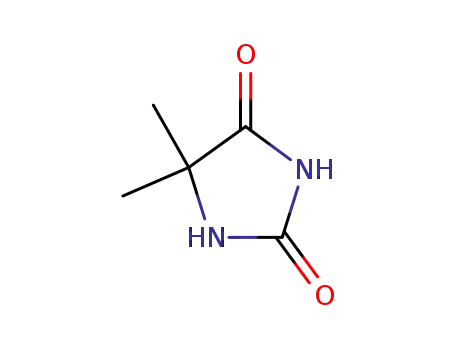
5,5-dimethyl-imidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
In water; at 120 ℃; for 0.533333h; under 15001.5 Torr; Flow reactor;
|
82% |
|
potassium cyanide; ammonium carbonate; acetone; In ethanol; water; at 55 - 60 ℃;
With hydrogenchloride; In ethanol; water;
|
65% |
|
acetone; With sodium metabisulfite;
potassium cyanide;
ammonium carbonate;
|
|
|
In ethanol; water; at 50 - 80 ℃; Reflux;
|
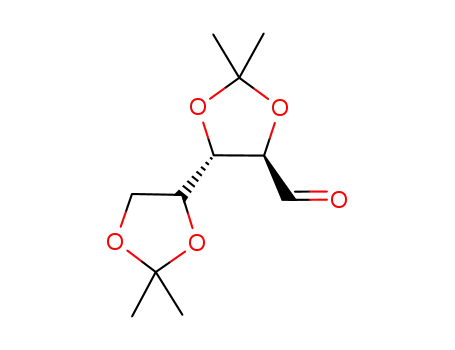
2,3:4,5-di-O-isopropylidene-D-xylose


5,5-dimethyl-imidazolidine-2,4-dione

![5-[(R)-2-((R)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-2-hydroxy-eth-(Z)-ylidene]-imidazolidine-2,4-dione](/upload/2024/11/fbeb39f7-b11c-4257-b421-62441e8e54ca.png)
5-[(R)-2-((R)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-2-hydroxy-eth-(Z)-ylidene]-imidazolidine-2,4-dione
| Conditions | Yield |
|---|---|
|
With potassium cyanide; In ethanol; water; at 50 ℃; for 24h;
|
62.5% |
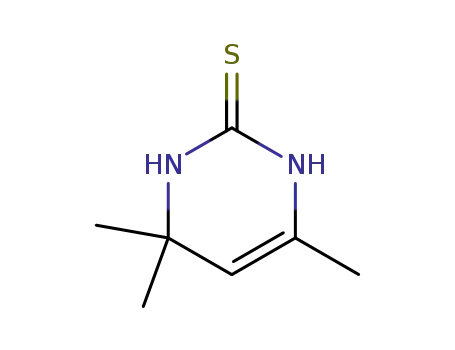
3,4-dihydro-4,4,6-trimethylpyrimidine-2(1H)-thione
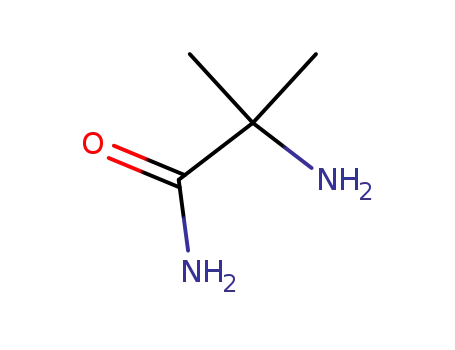
2-amino-2-methylpropanamide
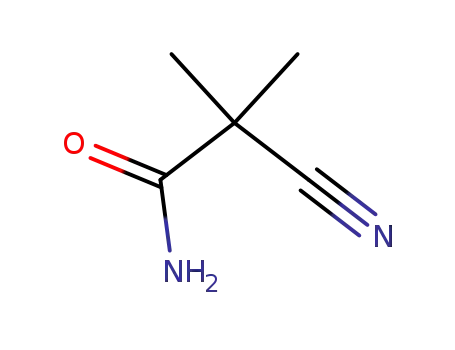
2-cyano-2-methylpropanamide
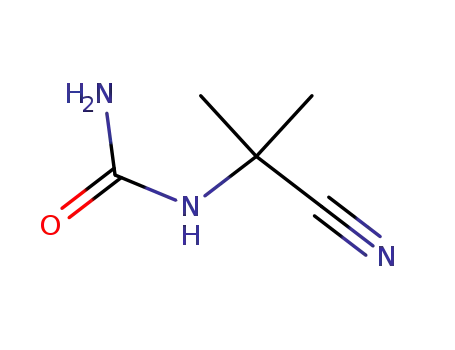
(1-Cyan-1-methylethyl)harnstoff
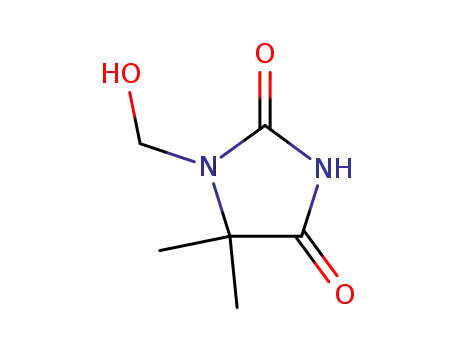
1-(hydroxymethyl)-5,5-dimethyl hydantoin
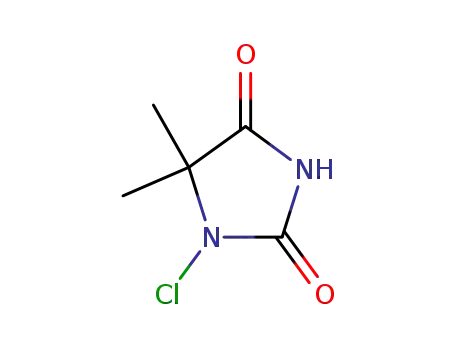
1-chloro-5,5-dimethylhydantoin
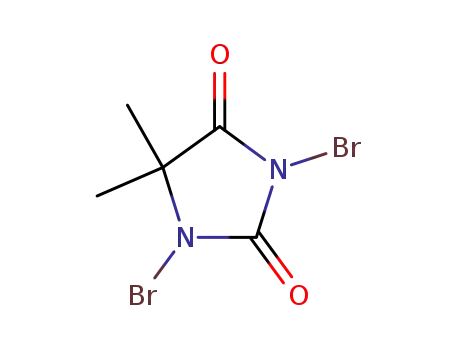
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
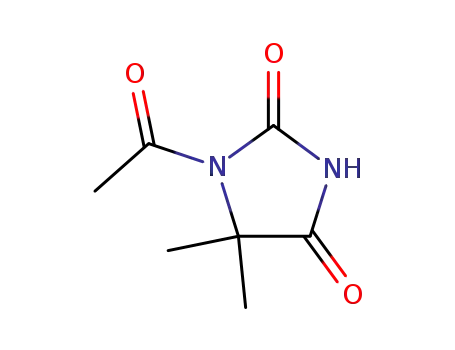
1-acetyl-5,5-dimethylhydantoin
CAS:123-00-2
CAS:1704-62-7
CAS:3030-47-5
CAS:111-26-2