
Product Details
| Product Name | Hexylamine |
| Alias | 1-Aminohexane;1-hexanamine;hexan-1-amine |
| English | |
| Molecular formula | C6H15N |
| Molecular weight | 101.19 |
| CAS No | 111-26-2 |
| EINECS | |
| Specification | Assay(GC)99% Moisture content < 0.3% color < 30 # (APHA) |
| Appearance traits | |
| Use | It Mainly used as the intermediate of dye, pigment, surfactant and pharmaceutical synthesis. |
| Package | 160kg/drum |
| Other product information | Properties: Appearance:Colorless liquid with ammonia flavor, Melting point:−23 °C(lit.) Boiling point:131 °C(lit.) Density:0.766 g/ml at 25 °C(lit.) Refractive index:N20/D 1.422(lit.) Flash(ing) point:8.9°C Water soluble properties:14 g/L (20 ºc) Sensibility:Air sensitive |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 55, p. 2271, 1990 DOI: 10.1021/jo00295a002Synthesis, p. 605, 1981 |
|
Air & Water Reactions |
Highly flammable. Slightly soluble in water. |
|
Reactivity Profile |
HEXYLAMINE neutralize acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
|
Flammability and Explosibility |
Flammable |
|
Purification Methods |
Dry with, and fractionally distil the hexylamine from, KOH or CaH2. Store away from CO2. [Beilstein 4 IV 709.] |
|
Definition |
ChEBI: A 6-carbon primary aliphatic amine. |
|
Aroma threshold values |
High strength odor; fishy type, recommend smelling in a 0.10% solution or less |
|
General Description |
A water-white liquid with an amine-like odor. Flash point 85°F. Boiling point 132°F. Less dense than water and poorly soluble in swater. Hence floats on water. May be toxic by inhalation, ingestion or skin absorption. |
InChI:InChI=1/C6H15N/c1-2-3-4-5-6-7/h2-7H2,1H3/p+1
High-quality chemical talents, abundant product resources, a perfect supply chain, a reasonable organizational structure, and a scientific management model are the core competitiveness of our company's continuous development. Based on good credibility, cooperation, development, and win-win purpose, after years of efforts, the company has established stable cooperative relationships with more than hundreds of chemical enterprises in domestic and established a good reputation and corporate image in the chemical field.
Tungsten monocarbide (WC) obtained throu...
Herein, we report on the use of nanopall...
Using mesoporous N-doped carbons (NCs) d...
A magnetic personality: Commercially ava...
The reactions of primary amines with N,N...
A new and efficient photosensitized proc...
Fatty amine synthesis from renewable sou...
Chemical recycling provides a promising ...
Malondialdehyde and acetaldehyde react t...
The azides are reduced to amines in very...
Fatty amines represent an important clas...
The reduction of polar bonds, in particu...
The cyclic (alkyl) (amino) carbene chrom...
Developing mild and efficient catalytic ...
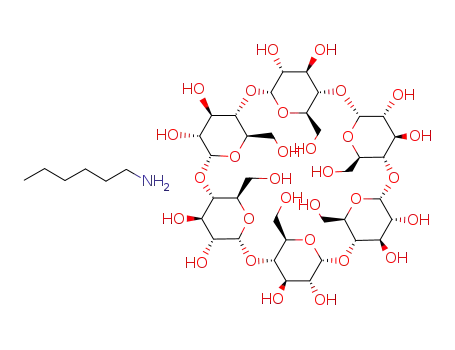
complex of α-cyclodextrin with n-hexylamine

hexan-1-amine

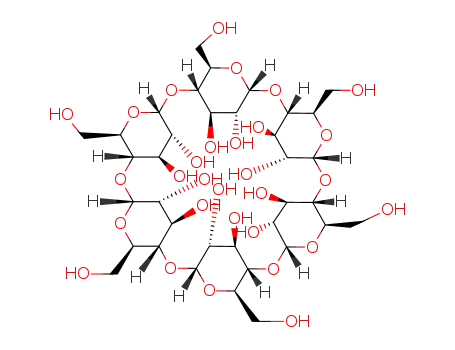
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
With phosphate buffer; In water-d2; at 25 ℃; Equilibrium constant; Thermodynamic data; standard molar enthalpy ΔrH0, standard molar Gibbs energy ΔrG0, standard molar entropy ΔrS0;
|
|
|
In alkaline aq. solution; at 25 ℃; pH=11.60; Equilibrium constant;
|
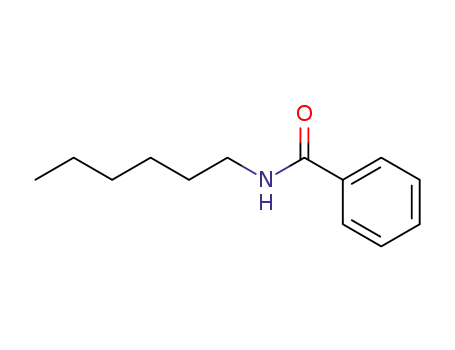
N-hexylbenzamide


hexan-1-amine

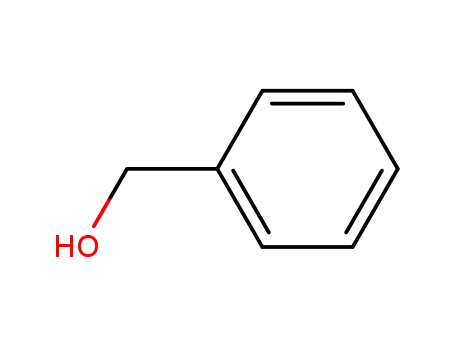
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With Ag/γ-Al2O3 (2.5 mol%); potassium tert-butylate; hydrogen; In 1,4-dioxane; at 150 ℃; for 48h; under 37503.8 Torr; chemoselective reaction; Green chemistry;
|
72 %Spectr. 70 %Spectr. |
|
With potassium tert-butylate; hydrogen; [Ru(PtBuNNHBn)H(CO)Cl]; In tetrahydrofuran; at 35 ℃; for 68h; under 7500.75 Torr;
|
63 %Chromat. 62 %Chromat. |
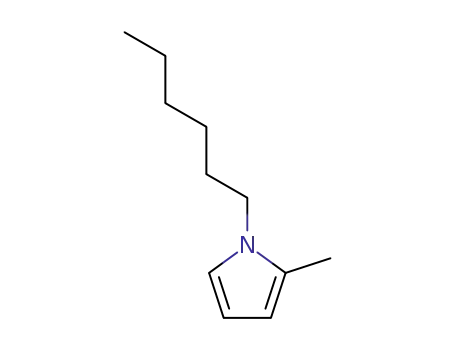
1-hexyl-2-methyl-pyrrole
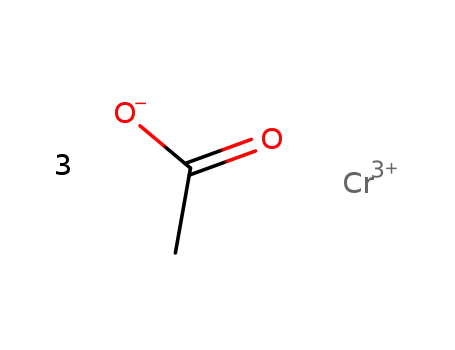
chromium(lll) acetate
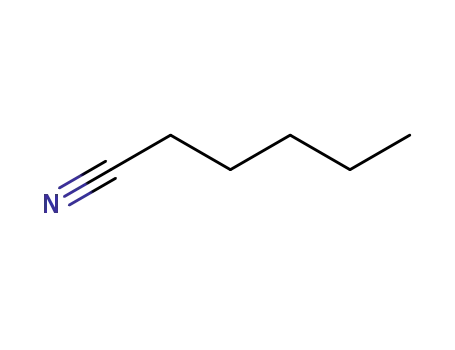
hexanenitrile
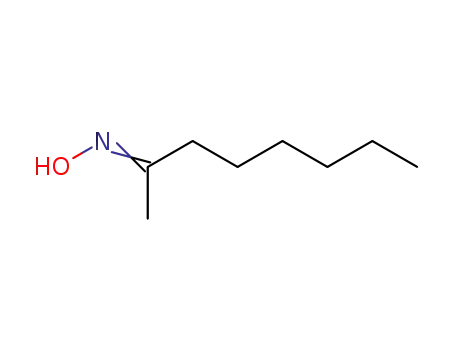
octan-2-one oxime
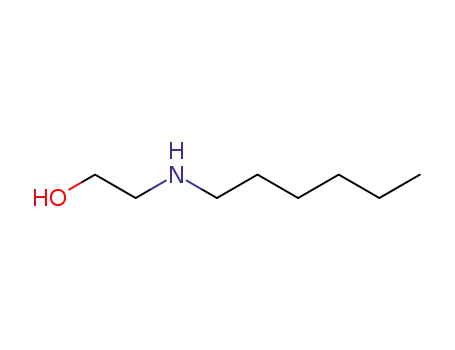
2-(hexylamino)ethan-1-ol
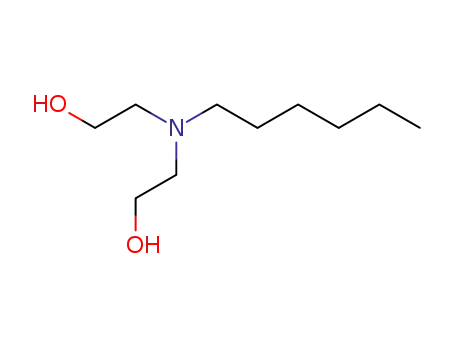
2-[hexyl-(2-hydroxyethyl)amino]ethanol
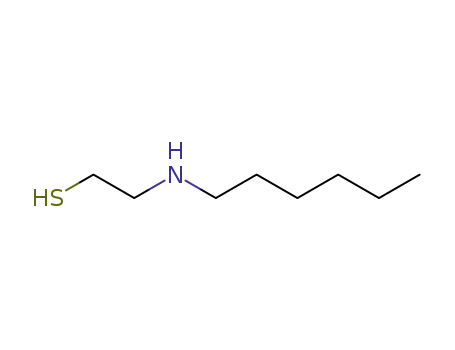
2-Hexylamino-ethylmercaptan
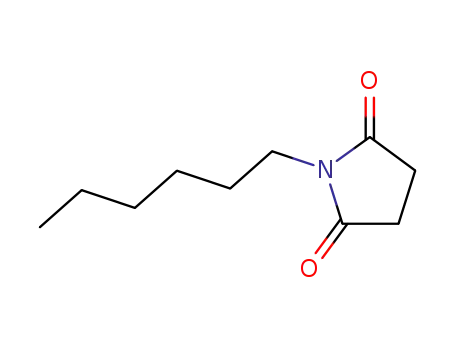
1-hexyl-2,5-pyrrolidinedione
CAS:80-70-6
CAS:10061-68-4
CAS:77-71-4
CAS:3855-32-1