Your Location:Home >Products >Inorganic chemicals >Rare light metals(Li,Rb,Cs,Be) >Lithium(Li) >16853-85-3

Product Details
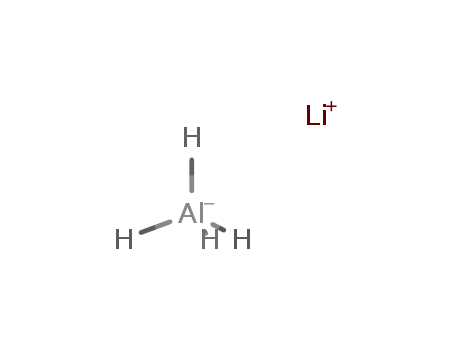
| Product Name | Lithium aluminium hydride |
| Alias | |
| English | |
| Molecular formula | LiAlH4 |
| Molecular weight | 37.95 |
| CAS No | 16853-85-3 |
| EINECS | 240-877-9 |
| Specification | 97% |
| Appearance traits | White or white crystalline powder, stable in dry air at room temperature. |
| Use | Carbonyl reagent. Reducing agent. Manufacture of other hydride and silane borane, etc.. In medicine, spices, pesticides, dyes and other fine organic synthesis used as a reducing agent |
| Package | 100g, 500g,1KG can |
| Other product information |
| General Description | A reactive white powder that may turn grey upon exposure. Can ignite upon friction when spread over large surfaces. Utilized in the production of other chemicals, as a polymerization catalyst, hydrogen source, and propellant. |
|
Preparation |
Lithium aluminum hydride is prepared by reaction of lithium hydride with aluminum chloride in diethylether: 4LiH + AlCl3 →(C2H5)2O→LiAlH4+3LiCl |
|
Uses |
Lithium aluminum hydride (LiAlH4) Reduces esters, carboxylic acids, acyl chlorides, aldehydes, epoxides, and ketones into alcohols. It is also used in the synthesis of reduced graphene oxide (rGO). LAH combined with metallic iron (Fe⁰) facilitates the hydrogenation of multi-substituted alkenes and arenes. |
| Air and Water Reactions | Reacts violently with water or moist air, leading to ignition of the evolved hydrogen. |
| Reactivity Profile | Highly reactive with oxidizing agents, hydroxy compounds, and some ethers. Reduces CO₂ and bicarbonates to methane and ethane at elevated temperatures. Forms explosive complexes with ether, dimethylamine, and tetrazoles. |
InChI:InChI=1/Al.Li.4H/q-1;+1;;;;/rAlH4.Li/h1H4;/q-1;+1
High quality chemical talents, abundant product resources, perfect supply chain, reasonable organizational structure and scientific management model are the core competitiveness of our company's continuous development. Based on good credibility, cooperation, development, and win-win purpose, after years of efforts, the company has established stable cooperative relationships with more than hundreds of chemical enterprises in domestic and established a good reputation and corporate image in the chemical field.
In this work, we investigated the effects of nanoconfinement on the properties of lithium aluminium hydride (LiAlH4). Our study reveals that nanoconfined LiAlH4 releases hydrogen at much lower temperatures than feasible with bulk LiAlH4.
Alkenes that normally do not react with LiAlH4 (3-hexene, cyclohexene, 1-Me-cyclohexene), can be reduced to the corresponding alkanes by a mixture of LiAlH4 and Fe0 (the iron was activated by Metal-Vapour-Synthesis).
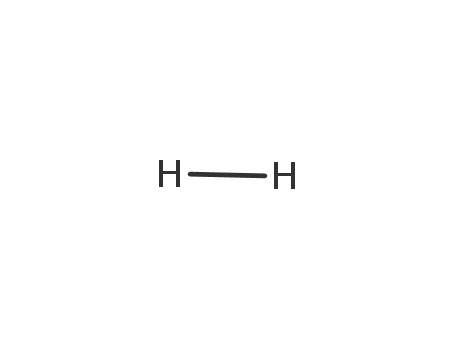
hydrogen


lithium hydride

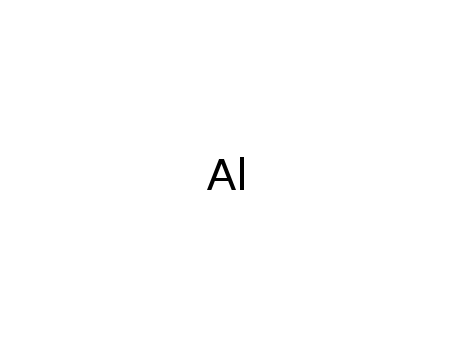
aluminium

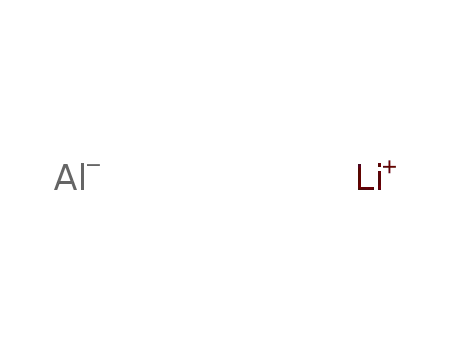
lithium aluminium tetrahydride
| Conditions | Yield |
|---|---|
|
titanium(III) chloride; In tetrahydrofuran; at 20 ℃; for 4h; under 73132.3 - 73162.7 Torr; Product distribution / selectivity; Balled milled;
|
|
|
With Et3Al; In toluene; temp. <= 150°C; pressure H2 <= 150 bar; pptn. toluene from diethyl ether soln.;
|
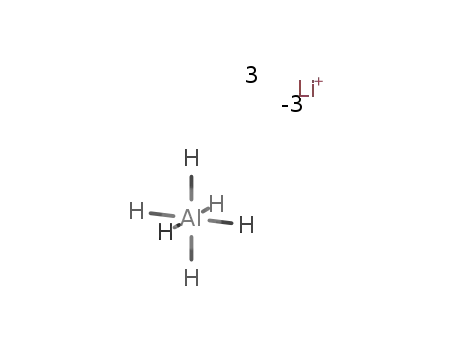
lithium hexahydroaluminate


hydrogen


lithium hydride


aluminium


lithium aluminium tetrahydride
| Conditions | Yield |
|---|---|
|
titanium; In diethyl ether; ball milling mixture of LiH, aluminium comd. and aluminium, obtained by dehydrogenating of LiAlH4, in hydrogen at different pressure for 2 h, addn. of ether, ball milling for 2 h at different hydrogen pressure at room temp.;
|
0% |
|
titanium; In neat (no solvent, solid phase); ball milling mixture of LiH, aluminium comd. and aluminium, obtained by dehydrogenating of LiAlH4, in hydrogen at 97.5 bar for 2 h; XRD analysis;
|
0% |
|
titanium; In tetrahydrofuran; ball milling mixture of LiH, aluminium comd. and aluminium, obtained by dehydrogenating of LiAlH4, in hydrogen at different pressure for 2 h, addn. of THF, ball miling for 2 h at different hydrogen pressure at room temp.; filtration, drying in vac. at 60°C for 6 h, XRD analysis;
|

hydrogen

aluminium
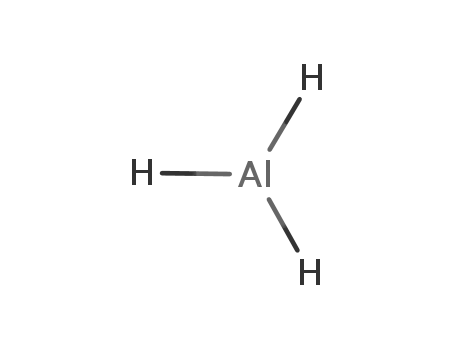
aluminium hydride
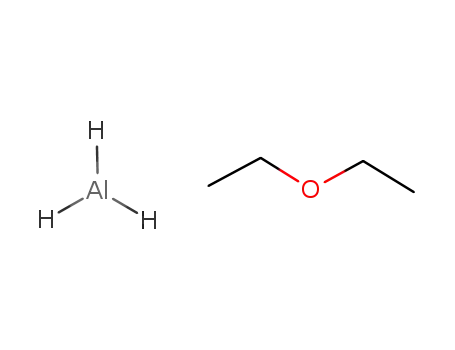
AlH3*Et2O
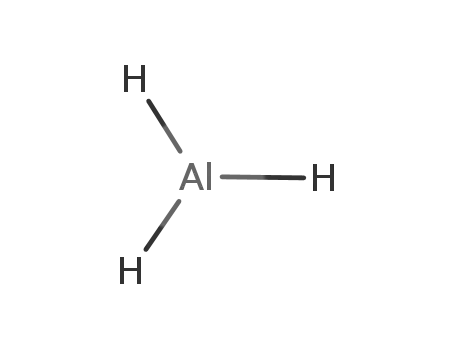
aluminium hydride
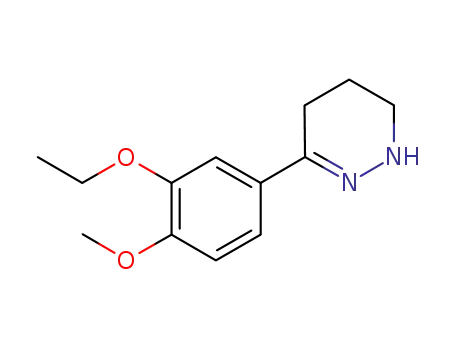
3-(3-ethoxy-4-methoxy-phenyl)-5,6-dihydro-4H-pyridazin
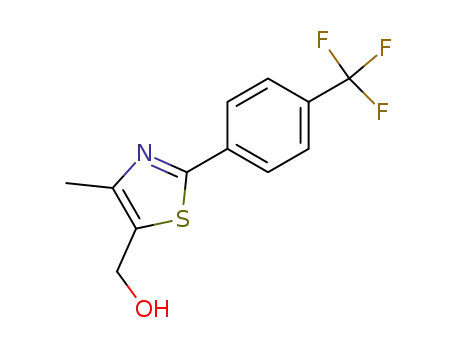
[4-methyl-2-(4-trifluoromethyl-phenyl)-thiazol-5-yl]-methanol
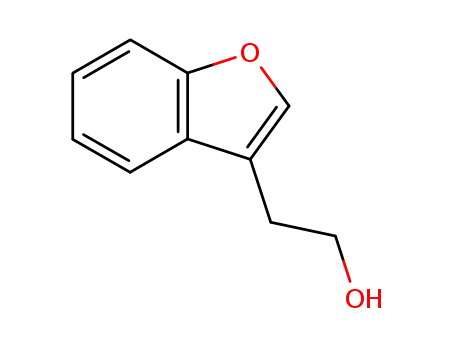
2-(benzofuran-3-yl)ethan-1-ol
CAS:7721-01-9
CAS:10294-54-9