
Product Details
| Product Name | Boron Oxide 99.999% | ||||||||||||||||||||||||||||||||||||||||
| Alias | diboron trioxide | ||||||||||||||||||||||||||||||||||||||||
| English | |||||||||||||||||||||||||||||||||||||||||
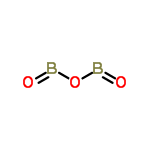
| Molecular formula | B2O3 |
||||||||||||||||||||||||||||||||||||||||
| Molecular weight | 69.62 | ||||||||||||||||||||||||||||||||||||||||
| CAS No | 1303-86-2 | ||||||||||||||||||||||||||||||||||||||||
| EINECS | 215-125-8 | ||||||||||||||||||||||||||||||||||||||||
| Specification |
|
||||||||||||||||||||||||||||||||||||||||
| Appearance traits | Colorless glass crystal or powder. The surface is greasy and tasteless. Melting point: 450 ° C (lit.); Boiling point: 1860 ° C density: 2.46 g/mL at 25 ° C (lit.); Solubility: soluble in acid, ethanol, hot water, slightly soluble in cold water. |
||||||||||||||||||||||||||||||||||||||||
| Use | Used as fluxing agent, also used in glass, enamel, manufacturing boron compound materials and color TV industry used as semiconductor silicon doping source and decomposition of silicate flux, also can be used in the manufacturing of boron |
||||||||||||||||||||||||||||||||||||||||
| Package | 1KG/bottle |
||||||||||||||||||||||||||||||||||||||||
| Other product information |
|
Physical properties |
Colorless glassy solid or vitreous crystal; hexagonal crystal system; slightly bitter taste; hygroscopic; density 2.55 g/cm3; melts at 450°C; vaporizes at 1,500°C; slightly soluble in cold water (3.3%), soluble in alcohol and boiling water (20%). |
|
Preparation |
Boric oxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750°C, the molten boric acid layer separates out from sodium sulfate. It then is decanted, cooled, and obtained in 96-97% purity. Boric acid above 99% purity may be obtained by fusing granular material. Boric oxide may be prepared by heating boric acid. 2B(OH)3 → B2O3 + 3H2O |
|
Production Methods |
Boric oxide is produced by thermal fusion of boric acid, forming a clear transparent glass-like solid that is subsequently ground into white vitreous granules. It is used principally in the manufacture of glass and vitreous products. |
|
General Description |
Colorless, semi-transparent glassy lumps or hard white odorless crystals. Mp 450°C; bp: 1860°C. Density: 2.46 g cm-3. Moderately soluble in water. Used as an insecticide; as the starting material for the synthesis of other boron compounds; as a fluxing agent in enamels and glasses; and in mixture with 2-6% boron nitride, as a bonding agent in the hot isostatic pressing of boron nitride ceramics. |
|
Reactivity Profile |
Boron oxide is non-combustible. Of generally low chemical reactivity. Reacts exothermically but slowly with water to form boric acid, a weak acid. Reacts exothermically with strong bases. May react with strong reducing agents such as metal hydrides, metal alkyls to generate flammable or explosive gases. May react violently on contact with bromine pentafluoride. Corrosive to metals in the presence of air. |
|
Hazard |
Eye and upper respiratory tract irritant. |
|
Health Hazard |
Boron oxide is an eye and respiratory irritant. In 113 workers exposed to boron oxide and boric acid dusts, there were statistically significant increases in symptoms of eye irrita- tion; dryness of the mouth, nose, and throat; sore throat; and productive cough compared with controls. The mean exposure level was 4.1mg/m3 , with a range of 1.2–8.5mg/m3 . Exposures may occasionally have exceeded 10 mg/m3 . Because of mixed exposures, the study does not indicate whether boron oxide or boric acid dust is more important in causing symp- toms, nor does it indicate the minimum duration of exposure necessary to produce symptoms. Excessive absorption of boron oxide may lead to cardiovascular collapse, alterations in temperature regulation, and coma. |
|
Biochem/physiol Actions |
Boric anhydride is an oxide of boron that shows antimicrobial property along with lower aminoalcohols. |
|
Potential Exposure |
Boron oxide is used in glass manufacture and the production of other boron compounds. It is used in fluxes, enamels, drying agents, and as a catalyst. |
|
Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required. |
|
Incompatibilities |
Incompatible with bromine pentafluoride, calcium oxide. Reacts slowly with water, forming boric acid. Reacts exothermically with alkaline material and strong bases. May react with strong reducing agents such as metal hydrides, metal alkyls to generate flammable or explosive gases. May react violently on contact with bromine pentafluoride. Corrosive to metals in the presence of air. |
InChI:InChI=1/B2O3/c3-1-5-2-4
Hangzhou Ocean Chemical Co., Ltd. is a chemical supplier that provides stable product quality, unique technical support, and high-quality service for global customers, Headquarters is located in Hangzhou with a superior entrepreneurial environment and business climate.
CAS:7550-35-8
CAS:74-96-4
CAS:3164-85-0
CAS:7440-64-4