Your Location:Home >10026-12-7
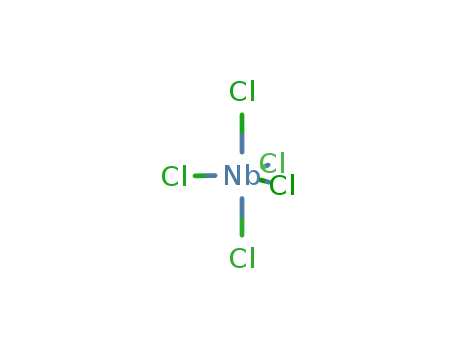

Product Details
| Product Name | Niobium(v) Chloride |
| Alias | Niobium Chloride, Pentachloroniobium |
| English | |
| Molecular formula | NbCl5 |
| Molecular weight | 270.4 |
| CAS No | 10026-12-7 |
| EINECS | 233-059-8 |
| Specification | 99% 99.9% |
| Appearance traits | White monoclinic crystal. |
| Use | Synthesis of a mixture of chlorine and aryl ether. |
| Package | 1KG/ bottles, 25KG/ barrels, or according to customer requirements |
| Other product information |
|
Physical properties |
Yellow monoclinic crystals; deliquesces; density 2.75 g/cm3; decomposes in moist air with the evolution of HCl; melts at 204.7°C; vaporizes at 254°C; decomposes in water; soluble in alcohol, hydrochloric acid, chloroform and carbon tetrachloride. |
|
Preparation |
Niobium pentachloride is obtained as an intermediate during extraction of niobium from its ores (see Niobium). Also, the pentachloride is obtained readily by direct chlorination of niobium metal at 300 to 350°C: 2Nb + 5Cl2 → 2NbCl5The pentachloride also may be made by chlorination of niobium pentoxide in the presence of carbon at 300°C. The products, however, contain small amounts of niobium oxide trichloride, NbOCl3. |
|
Hazard |
May evolve fumes of hydrogen chloride. Keep dry |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion. May cause kidney injury. When heated to decomposition it emits very toxic fumes of Cl-. See also NIOBIUM and CHLORIDES. |
|
Purification Methods |
It forms yellow, very deliquescent crystals which decompose in moist air to liberate HCl. It should be kept in a dry box flushed with N2 in the presence of P2O5. Wash it with CCl4 and dry it over P2O5. The yellow crystals usually contain a few small, dirty white pellets among the yellow needles. These should be easily picked out. Upon grinding in a dry box, however, they turn yellow. NbCl5 has been sublimed and fractionated in an electric furnace. [Epperson Inorg Synth VII 163 1963, Alexander & Fairbrother J Chem Soc suppl 233 1949.] d2 5 20 pK25 |
InChI:InChI=1/5ClH.Nb/h5*1H;/q;;;;;+2/p-5
High-quality chemical talents, abundant product resources, a perfect supply chain, a reasonable organizational structure, and a scientific management model are the core competitiveness of our company's continuous development. Based on good credibility, cooperation, development, and win-win purpose, after years of efforts, the company has established stable cooperative relationships with more than hundreds of chemical enterprises in domestic and established a good reputation and corporate image in the chemical field.
A series of oxidation-reduction and halo...
The reaction of chlorine with columbite ...
The equilibrium constant for the chloro ...
Niobium halides Nb3X8 (X=Cl, Br, I) are ...
The cyanomethane adduct of NbCl4 analyse...
The molecular structures of NbOBr3, NbSC...
Improved preparations leading to the com...
Thermogravimetry is proposed for studyin...
Black crystals of U2Ta6O19 with hexagona...
Metal nitride coatings are deposited eff...

niobium pentachloride

tert-butylamine
| Conditions | Yield |
|---|---|
|
|
79% |


niobium pentachloride
| Conditions | Yield |
|---|---|
|
|
phosgene

niobium(V) oxide chloride
chlorine
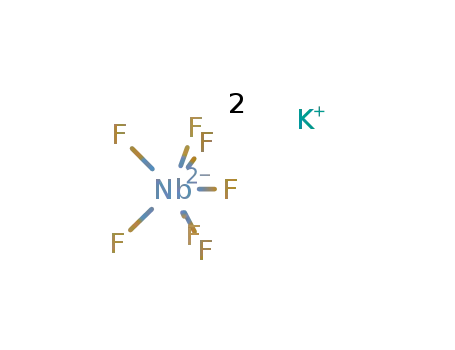
potassium heptafluoroniobate(V)
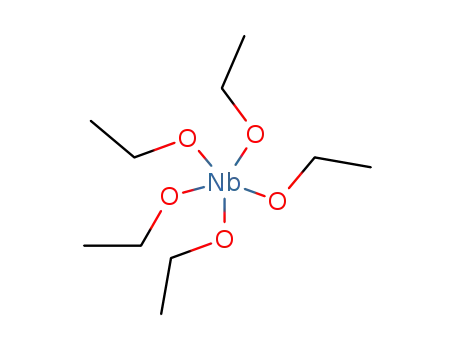
niobium (V) ethoxide
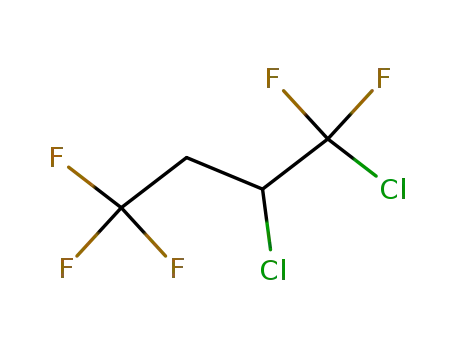
1,1,4,4,4-pentafluoro-1,2-dichlorobutane
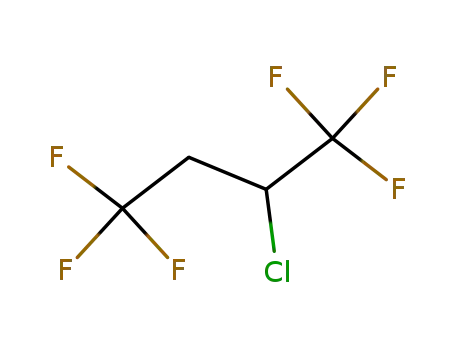
2-chloro-1,1,1,4,4,4-hexafluorobutane
1,1,1-trifluoro-2,2-dichloroethane
CAS:10024-93-8
CAS:18851-33-7
CAS:1120-24-7
CAS:5625-37-6